REVISION NOTES
IGCSE Edexcel Chemistry
1.2 Elements, Compounds & Mixtures
1.2.1 Understand how to classify a substance as an element, compound or mixture

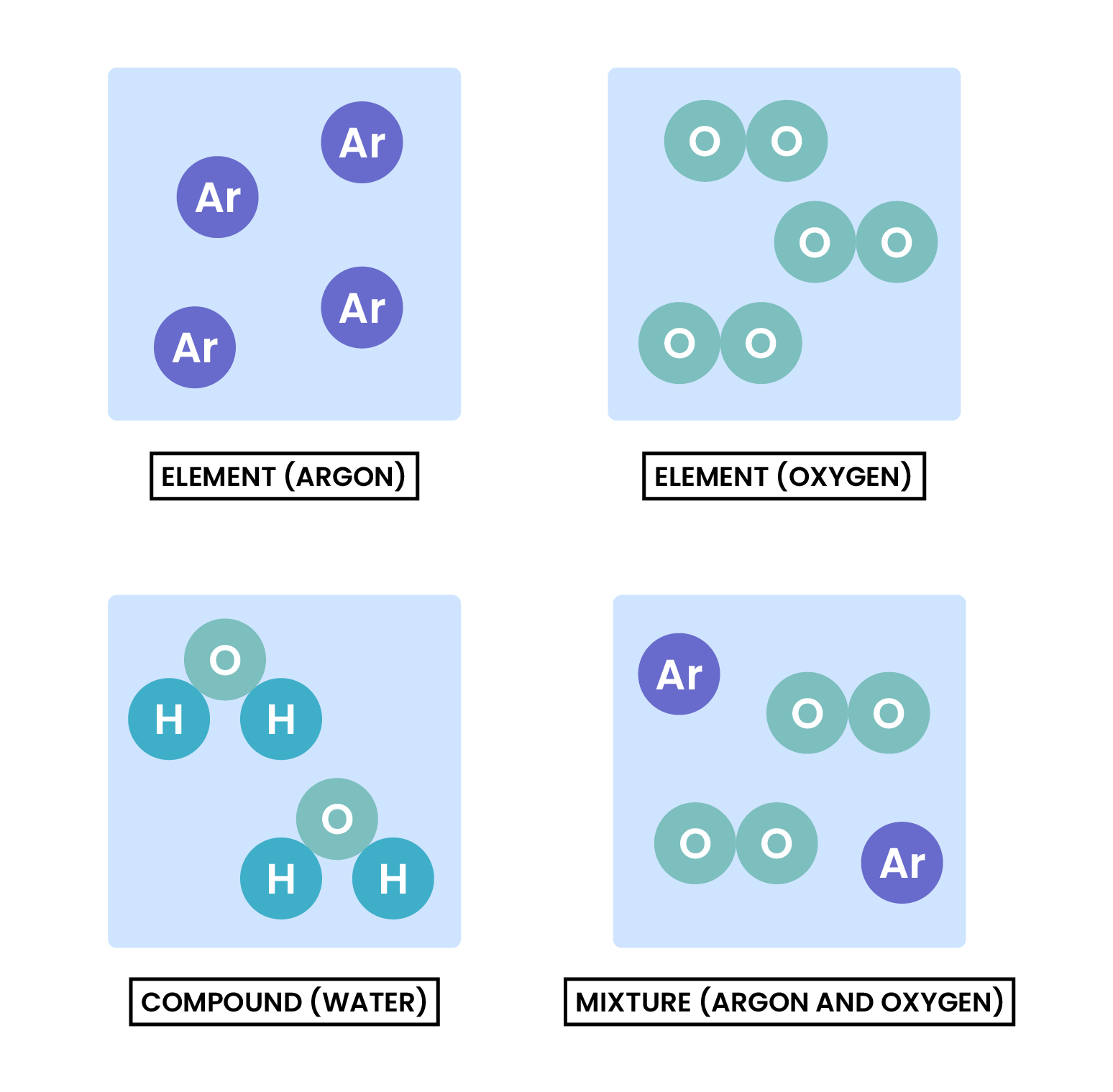
1.2.2 Understand that a pure substance has a fixed melting and boiling point, but that a mixture may melt or boil over a range of temperatures

1.2.3 describe these experimental techniques for the separation of mixtures:
- Simple distillation
- Factional distillation
- Filtration
- Crystallisation
- Paper chromatography
Summary of Separation Technique
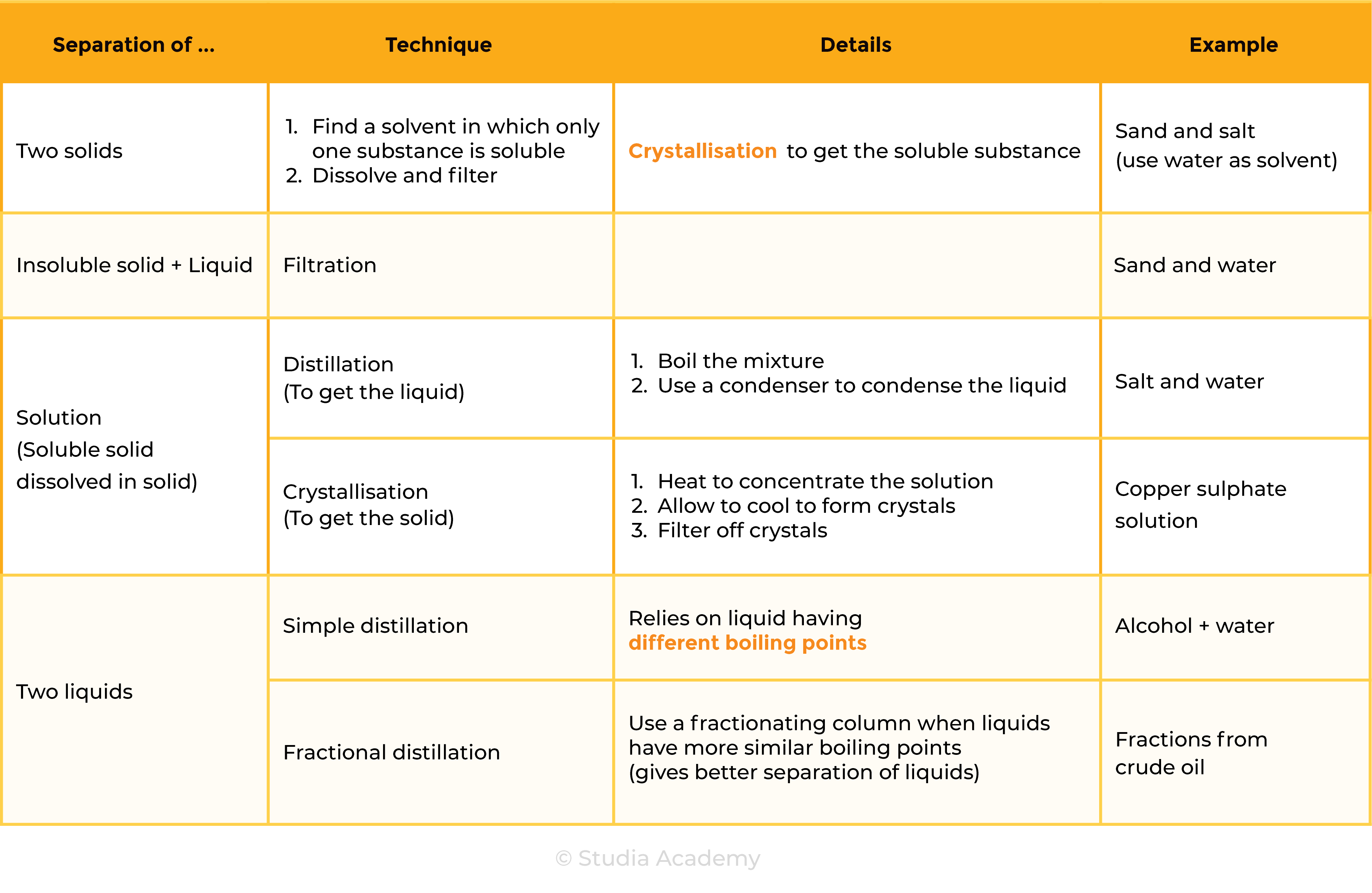
Filtration
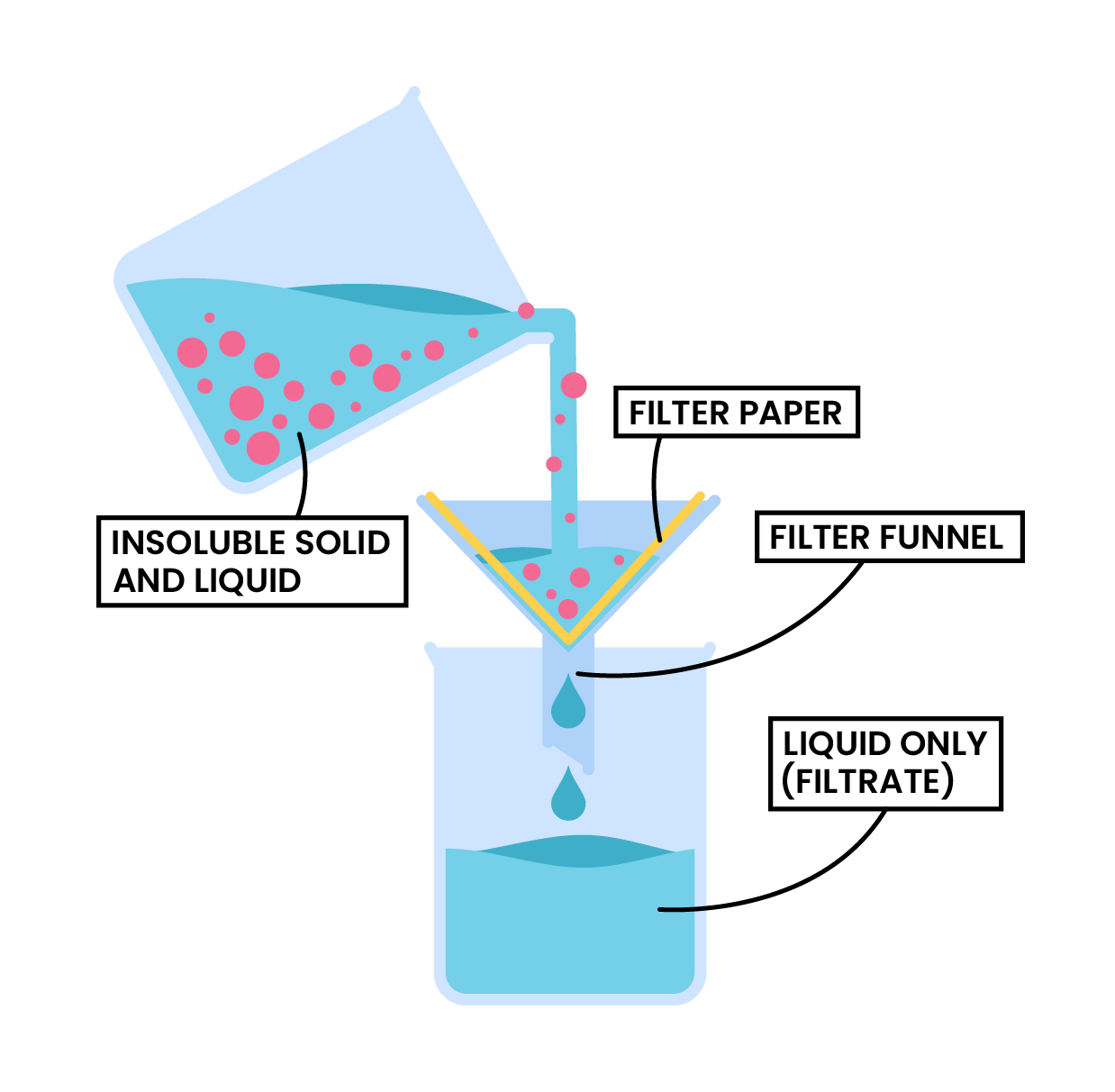
Crystallisation
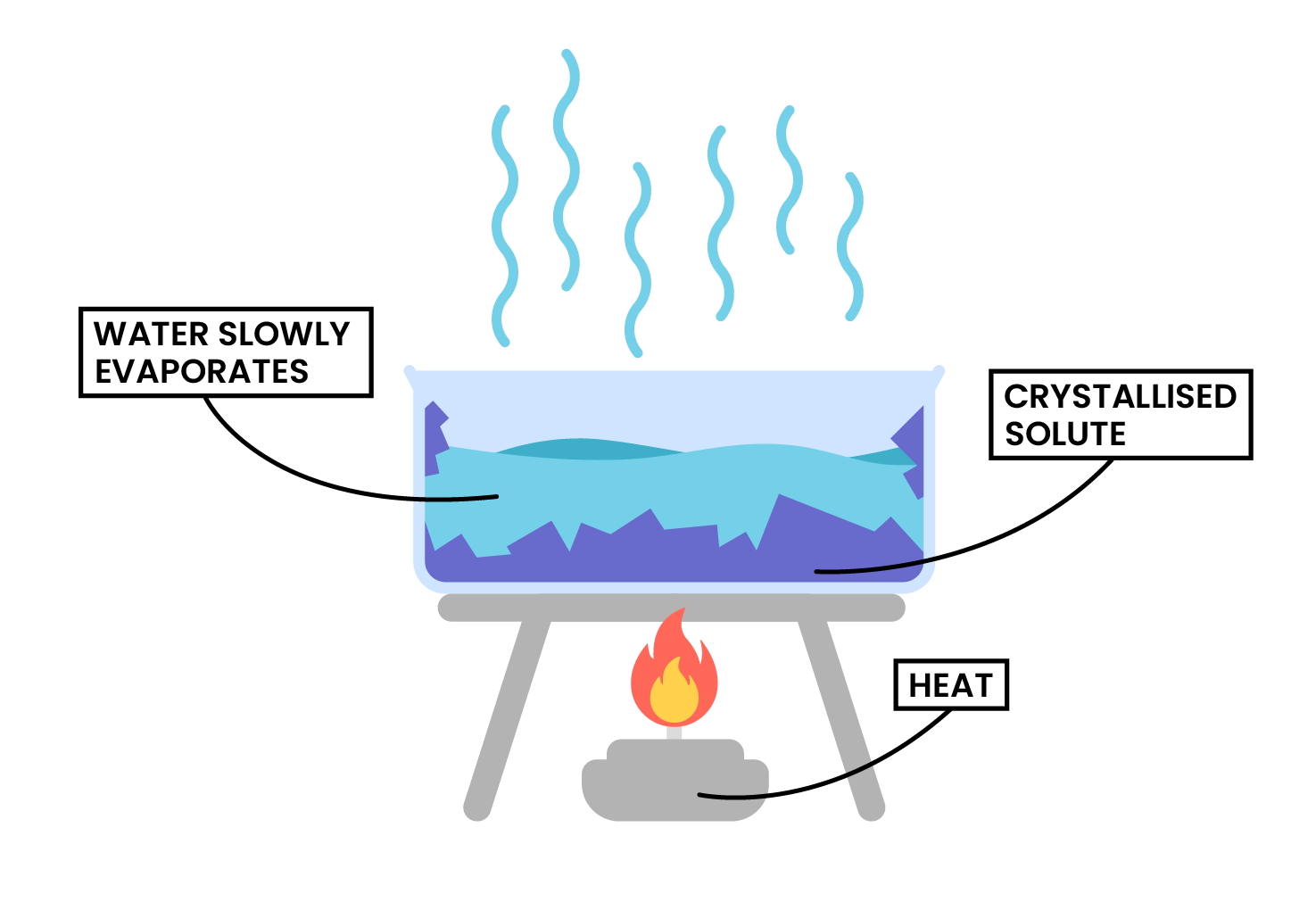
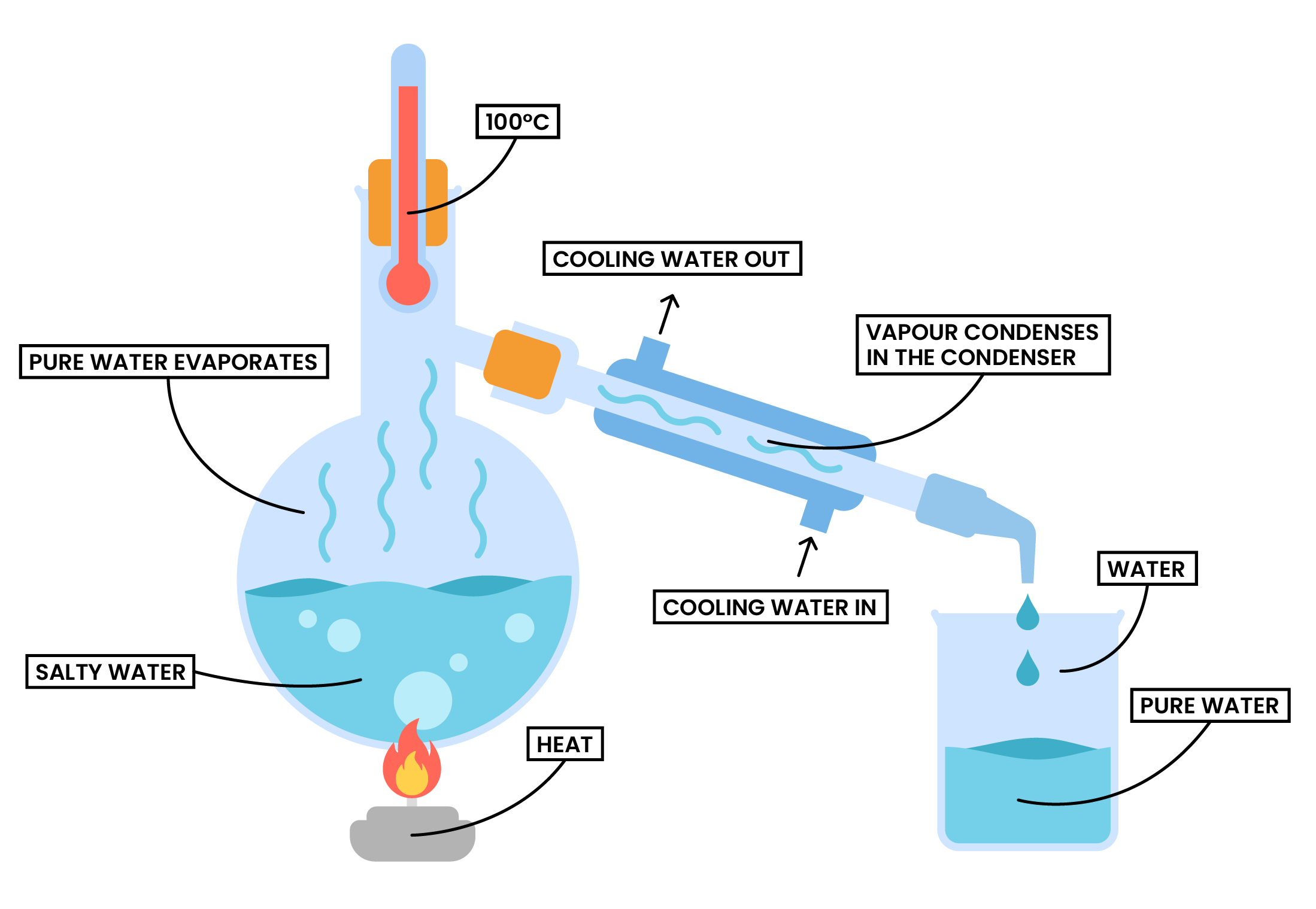
Simple Distillation
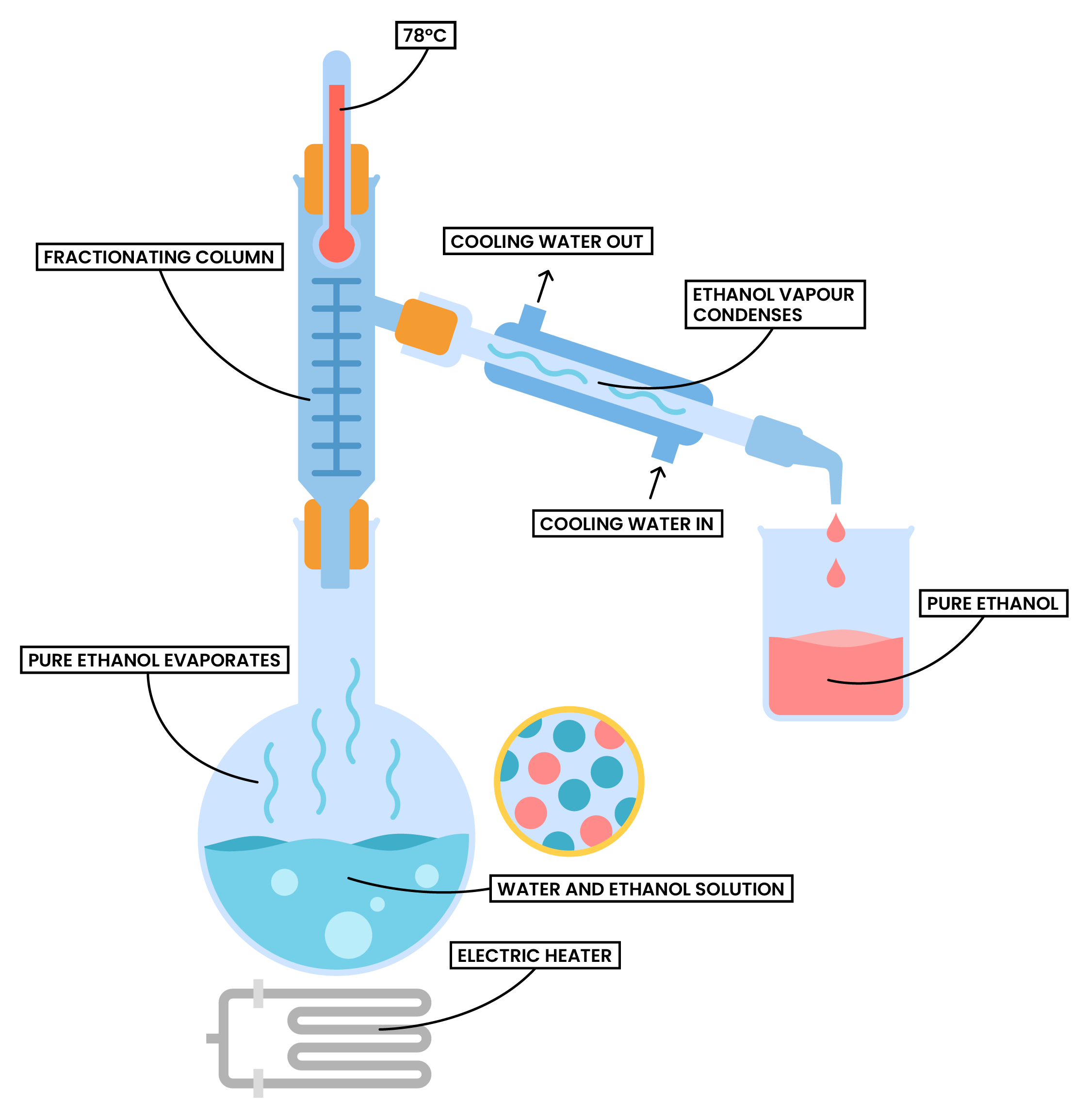
Fractional Distillation
PAPER CHROMATOGRAPHY
- This technique is used to separate substances that have different solubilities in a given solvent
- It can be used to identify the impurity of a sample
- Stationary phase: chromatography paper
- Mobile phase: solvent
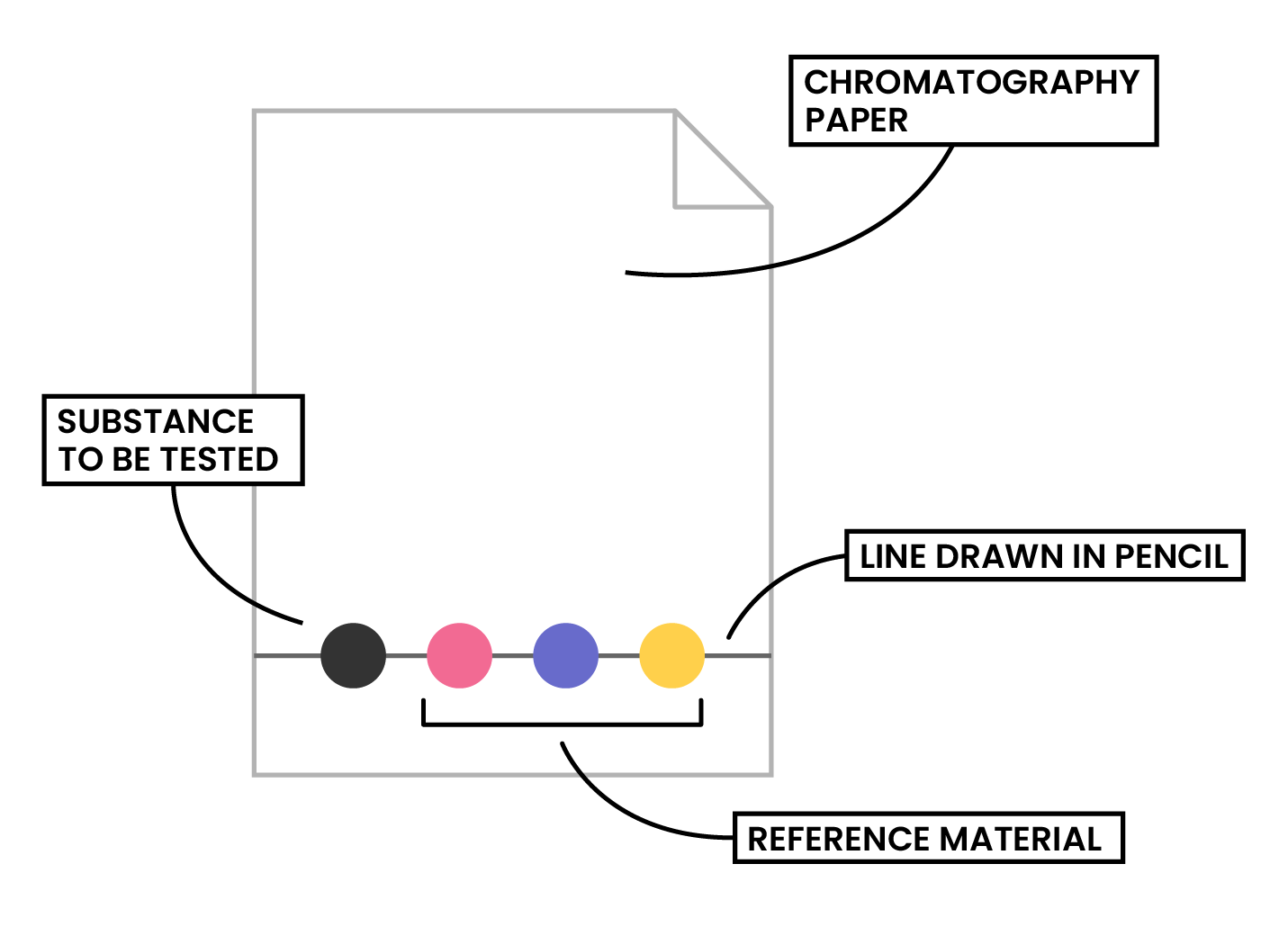
Step 1: A pencil line is drawn on chromatography paper
Step 2: Sample is spotted on the pencil line
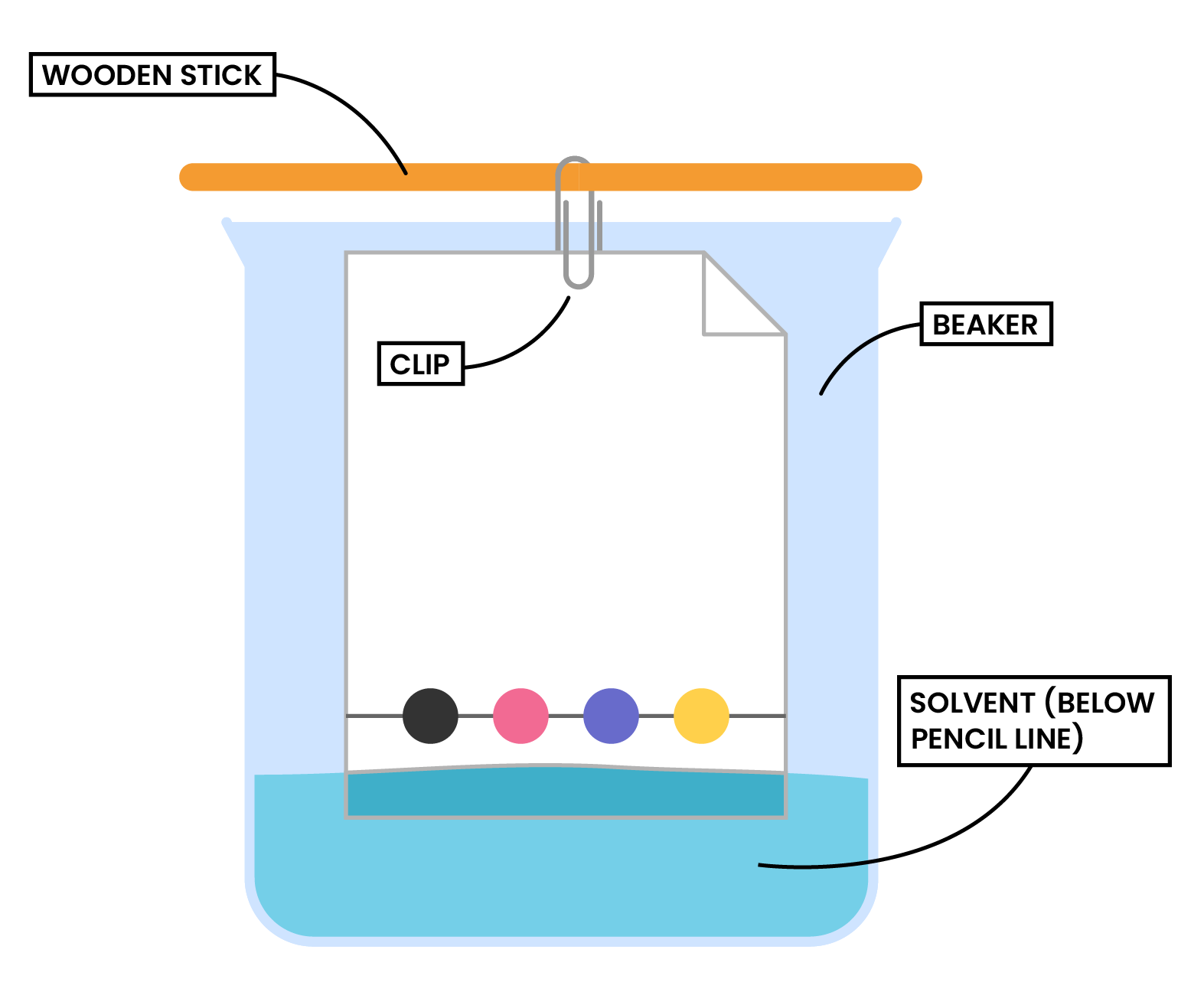
Step 3: The paper is then lowered into the solvent container (make sure that the pencil line sits above the level of the solvent)
Step 4: The solvent travels up the paper, taking some of the coloured substances with it
Step 5: Different substances have different solubilities so will travel at different rates, causing the substances to spread apart
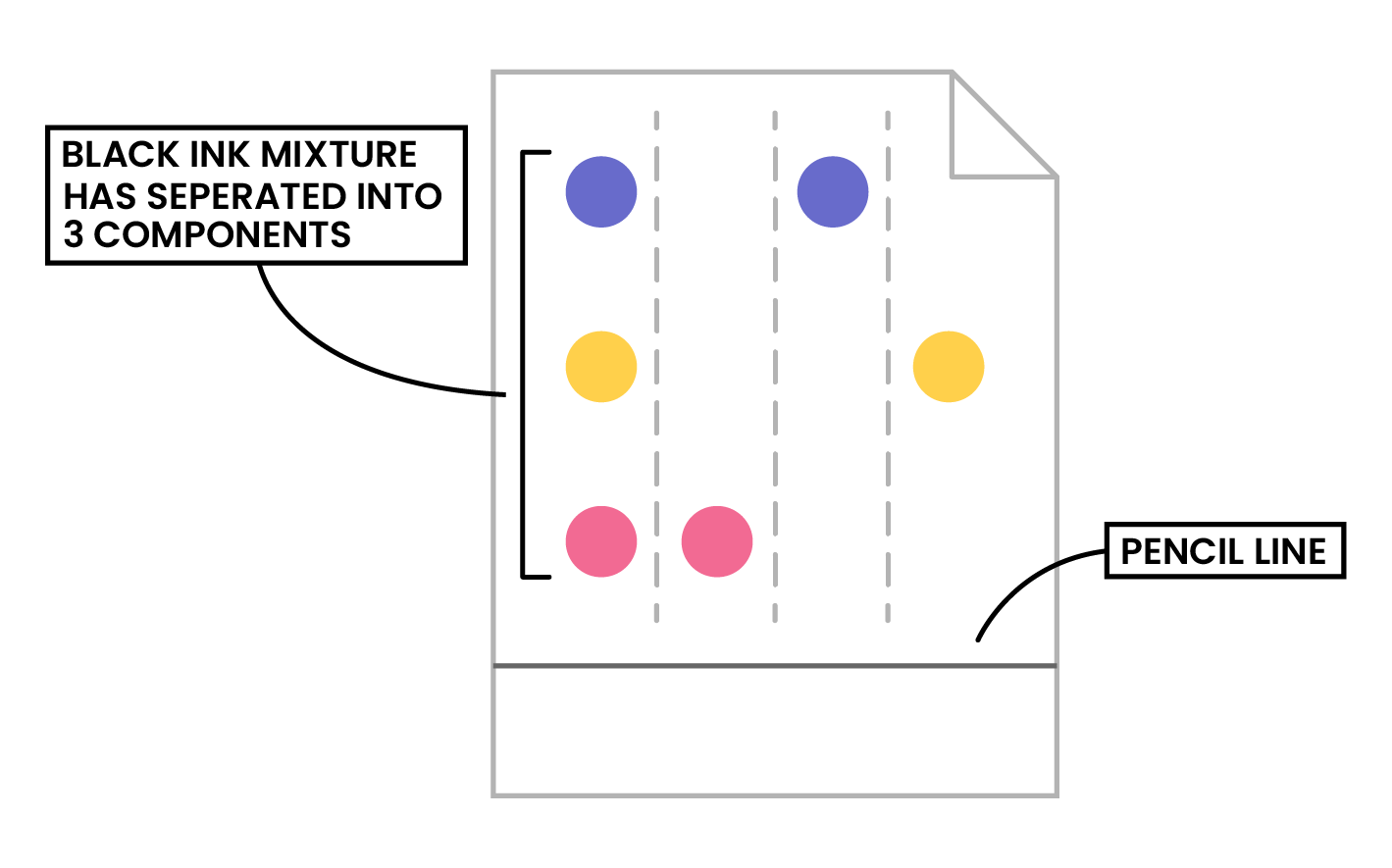
KEY POINTS
- Pencil is used as ink would run into the chromatogram along with the samples
- Make sure that the pencil line sits above the level of the solvent, so the samples don’t wash into the solvent container
- Substances with higher solubility will travel further than the others
1.2.4 Understand how a chromatogram provides information about the composition of a mixture
- If two or more substances are the same, they will produce identical chromatograms
- If the substance is a mixture, it will separate on the paper to show all the different components as separate spots
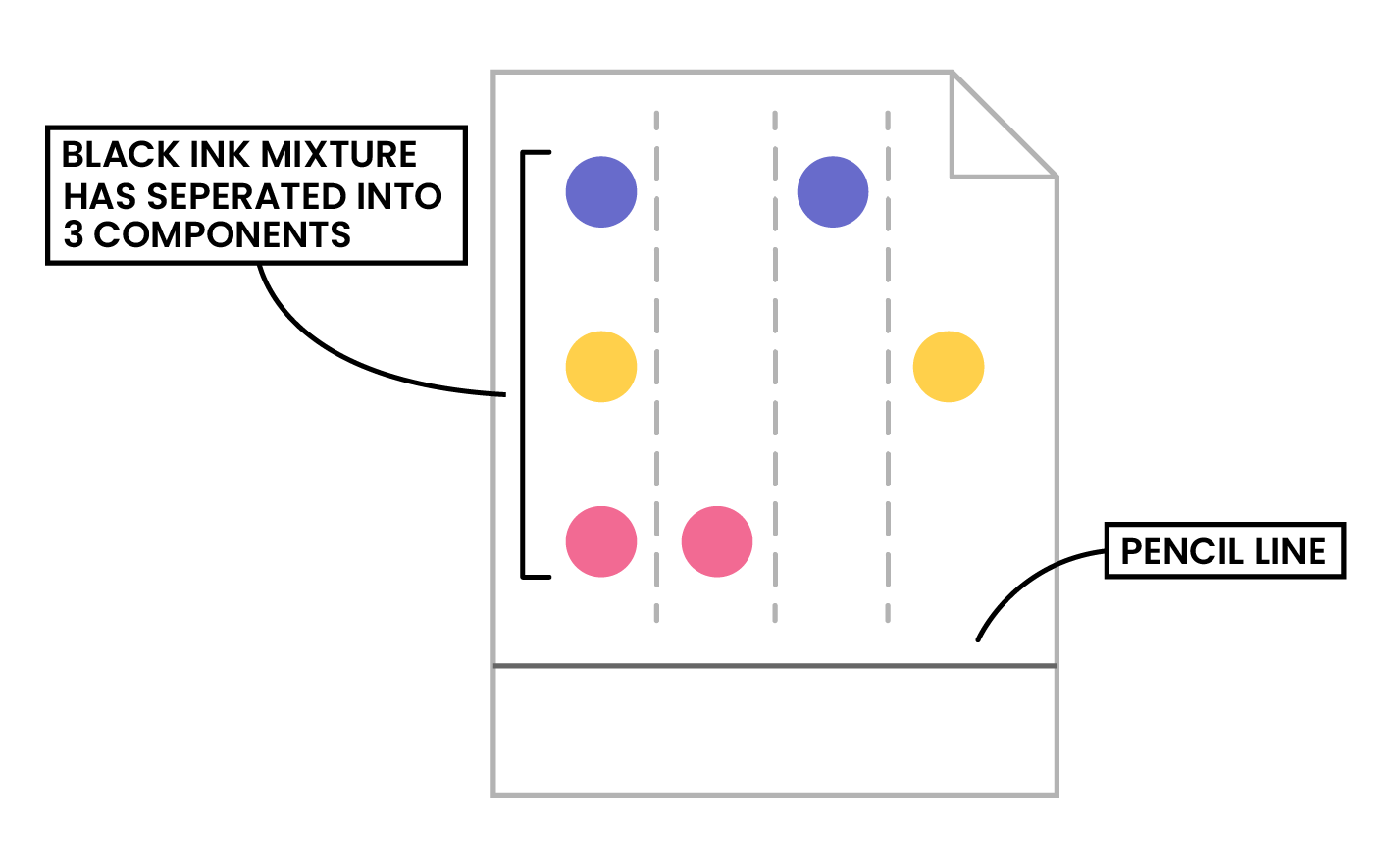
- The sample on the left would be a mixture as it is separated into three spots (three components)
- The samples on the right would be pure substance as they have only one spot (one component)
1.2.5 understand how to use the calculation of Rf values to identify the components of a mixture
RETENTION FACTOR (Rf) VALUE
- Used to identify the components of mixtures
- The Rf value of a particular compound is always the same
- But it depends on the solvent used
- Calculating the Rf value allows chemists to identify unknown substances
- By comparing the Rf values with the reference values of known substances under the same conditions
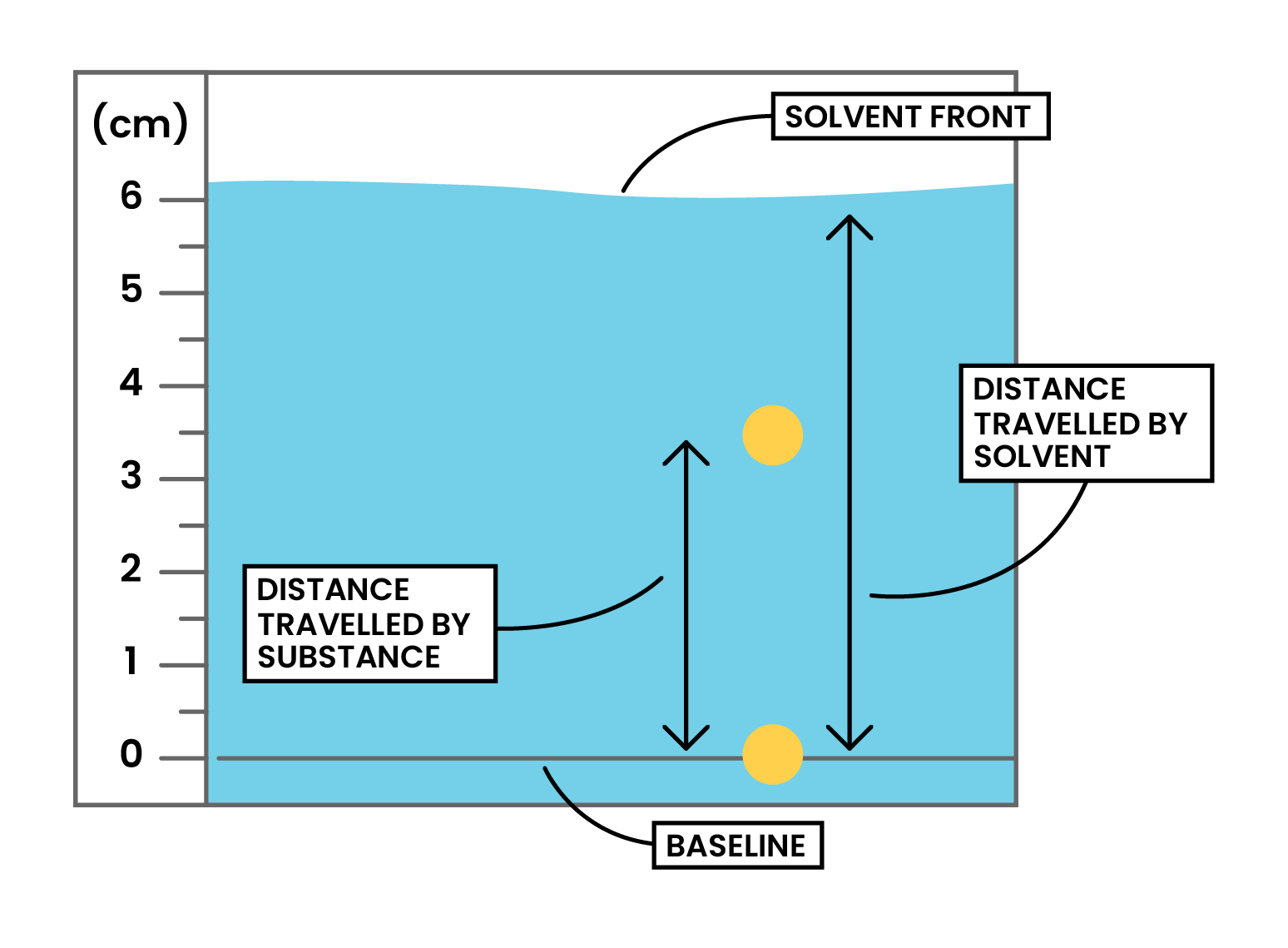
Calculation
- Always lie between 0 and 1
- The closer it is to 1, the more soluble is that component in the solvent
- The Rf value is a ratio and therefore has no units
1.2.6 Practical: investigate paper chromatography using inks/food colourings
AIM
- Investigate how paper chromatography can be used to separate and identify a mixture of food colourings
EQUIPMENT LIST
- A 250 cm3 beaker
- A wooden spill
- A rectangle of chromatography paper
- Five glass capillary tubes
- Ruler & pencil
METHOD
- Draw start line in pencil
- Place samples on the start line
- Lower the paper into a beaker of solvent with start line above the solvent
- Allow the solvent to travel at least three quarters of the way up the paper
- Remove the paper when solvent almost reaches the top
- Marke solvent front
- Allow to dry
- Measure the distance from the start line to the solvent front line
- Measure the distance from the start line to each separated component
Result Table
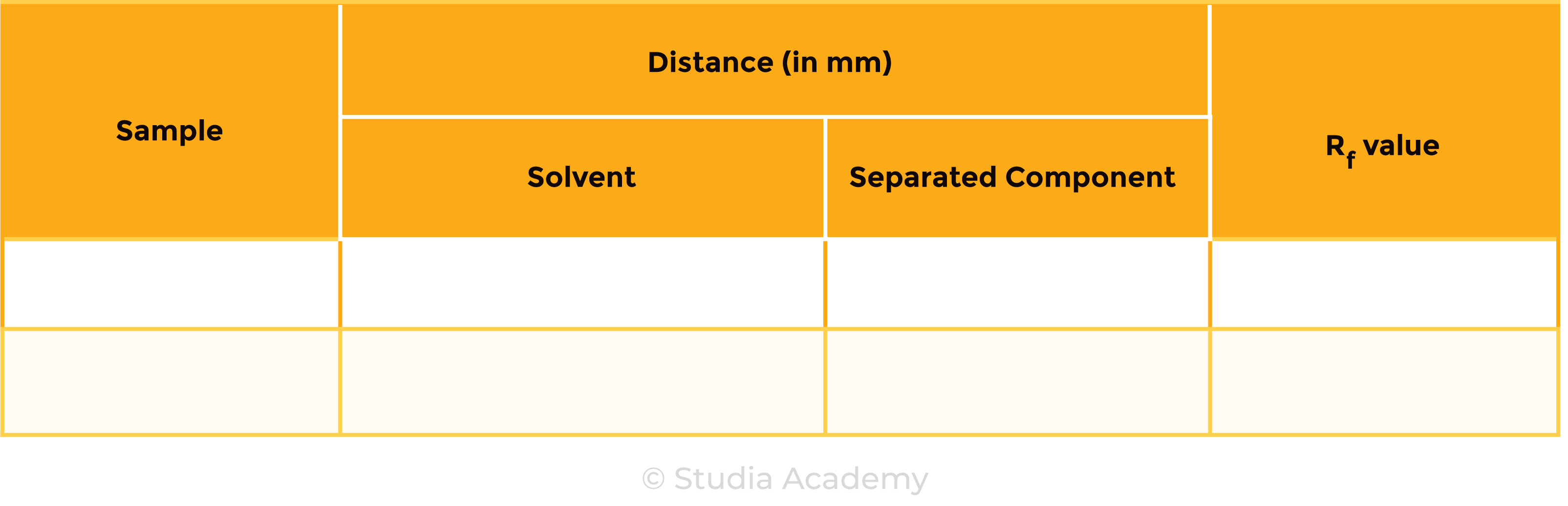
RESULTS
- As solvent travels up the paper, different substances have different solubilities so will travel at different rates, causing substances to spread apart
- This will show the different components of the ink/colouring

