REVISION NOTES
IGCSE Edexcel Chemistry
1.9 Electrolysis
1.9.1C Understand why covalent compounds do not conduct electricity
Electricity is a flow of charged particles (e.g. ions, electrons)
Covalent compounds do not conduct electricity because the electrons are fixed in the covalent bonds
1.9.2C Understand why ionic compounds conduct electricity only when molten or in aqueous solution
Ions are fixed when ionic compounds are solid, meaning they can’t move so can’t conduct electricity.
However, when the compounds are molten or in aqueous solution, the ions (that are electrically charged) are able to move and carry charge.
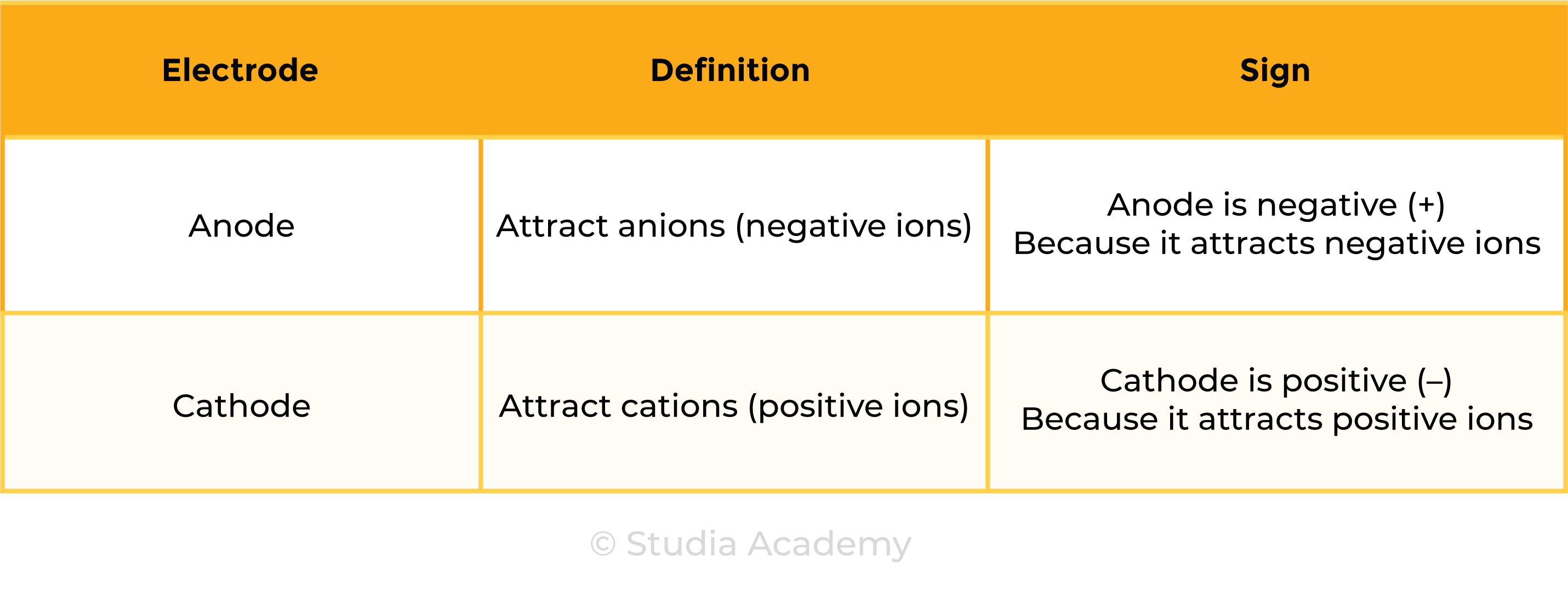
1.9.3C Know that anion and cation are terms used to refer to negative and positive ions respectively
Cations: positively charged ions (+) due to loss of electrons
Anions: negatively charged ions (-) due to gain of electrons
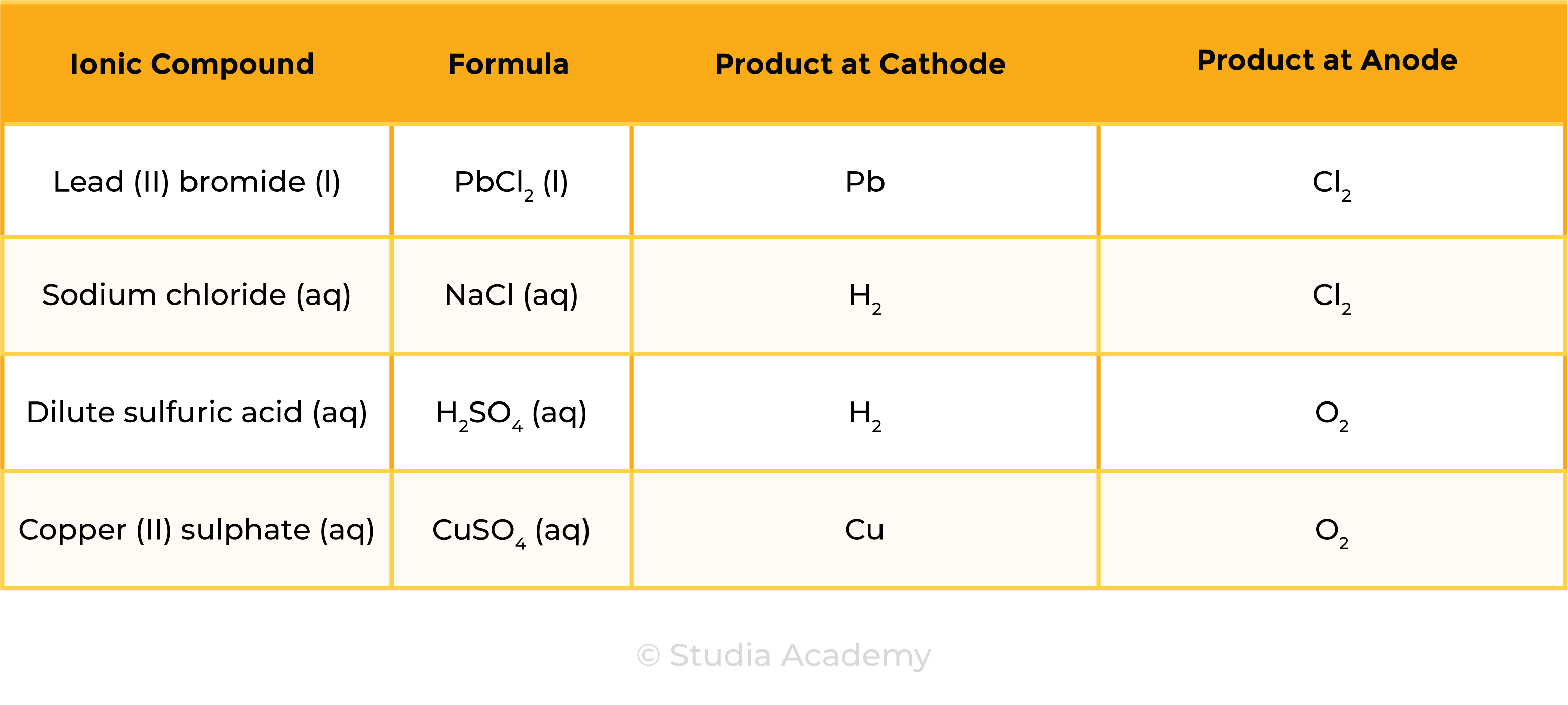
1.9.4C Describe experiments to investigate electrolysis, using inert electrodes, of molten compounds (including lead(II) bromide) and aqueous solutions (including sodium chloride, dilute sulfuric acid and copper(II) sulfate) and to predict the products
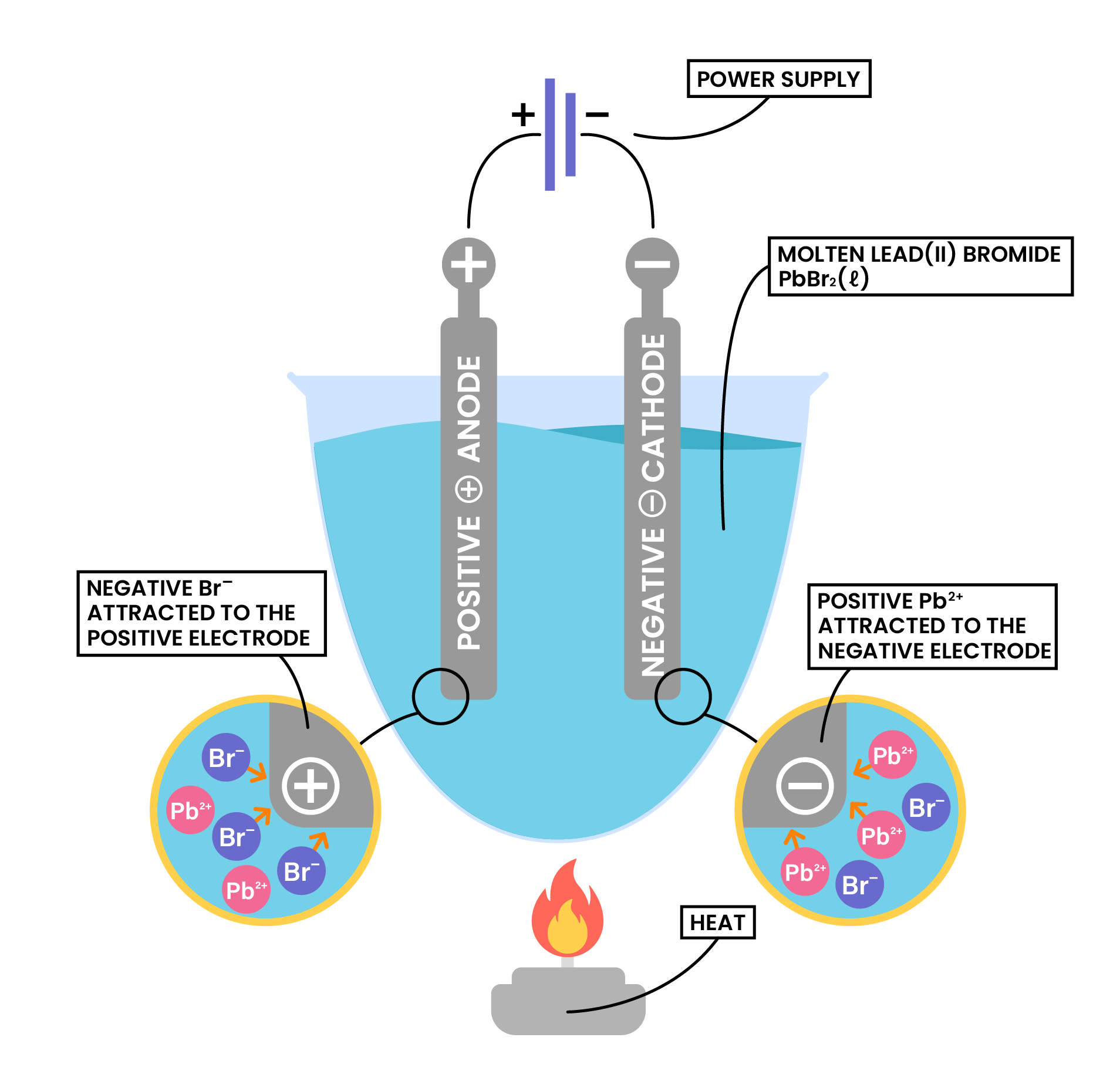
ELECTROLYSIS
- Breakdown of ionic compounds in molten or aqueous states using electricity to form new products
- The setup consists of:
- Electrodes (anode & cathode)
- Power source
- External wires
- Beaker
- Electrolytic solution (electrolyte)
Procedure
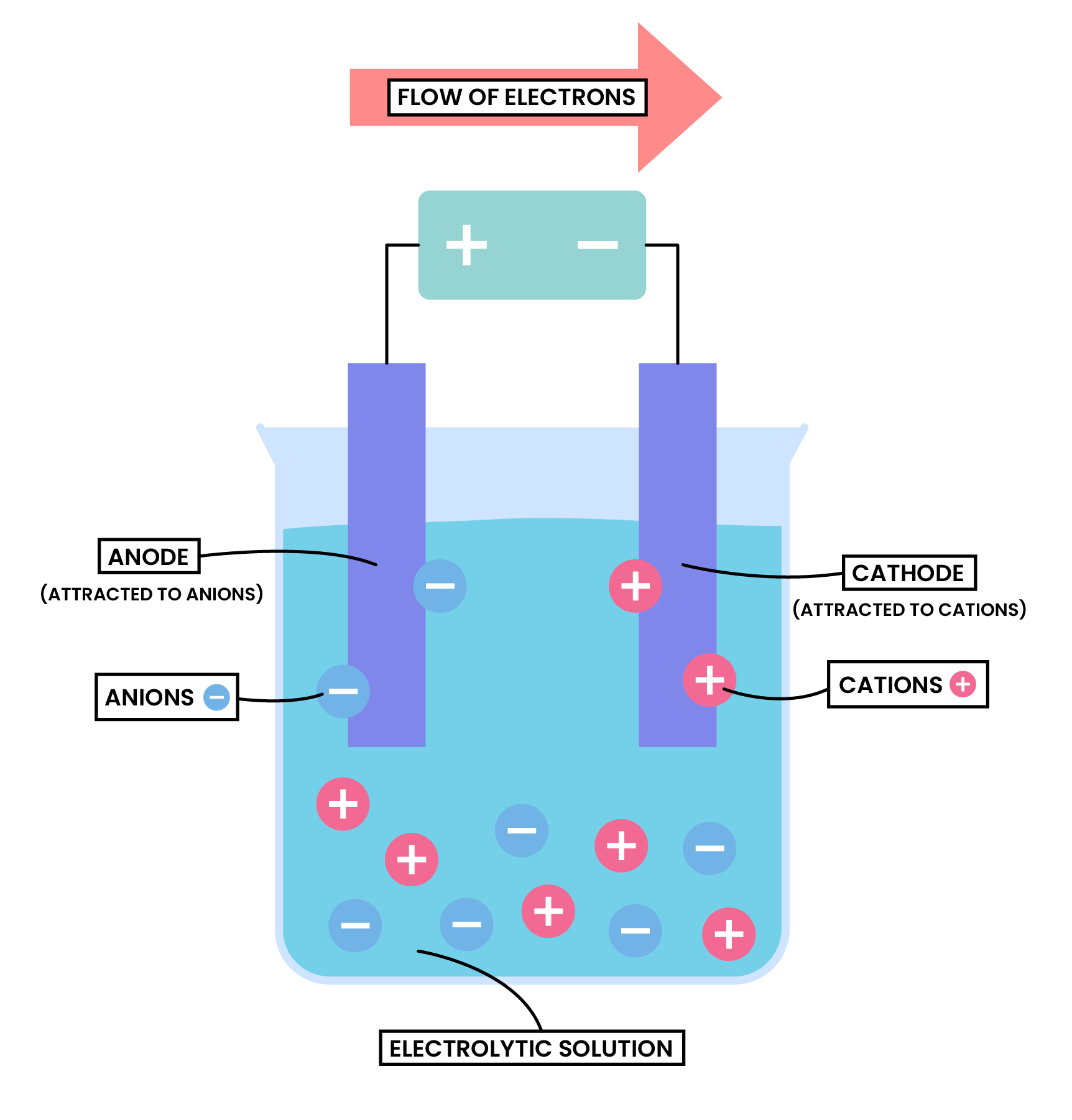
- Anions approach anode and lose electrons
- Electrons are passed from anode to cathode through the wire and power supply
- Cations approach cathode and accept the incoming electrons
Power supply
- Electrons always flow in from the positive end of the battery, and come out from the negative end
- Therefore, anode is always at the positive end of the battery, cathode is always at the negative end of the battery
PRODUCTS OF ELECTROLYSIS
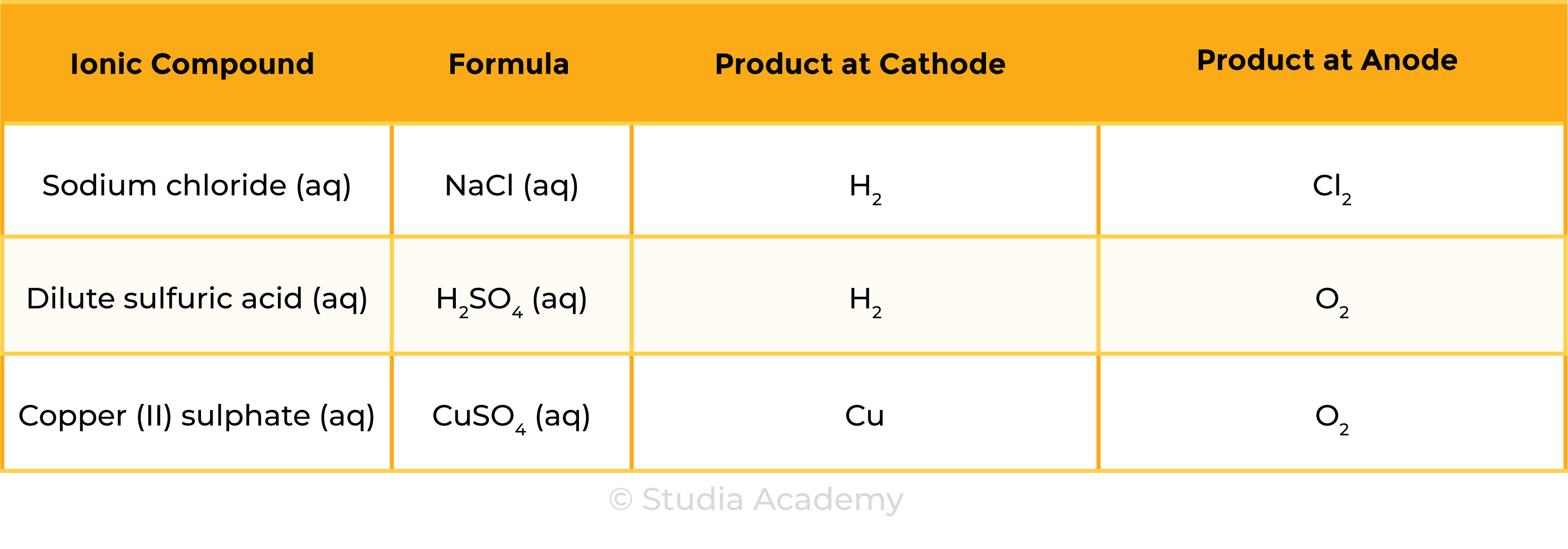
- The products of electrolysis depend on the ionic compound and its state (molten or aqueous)
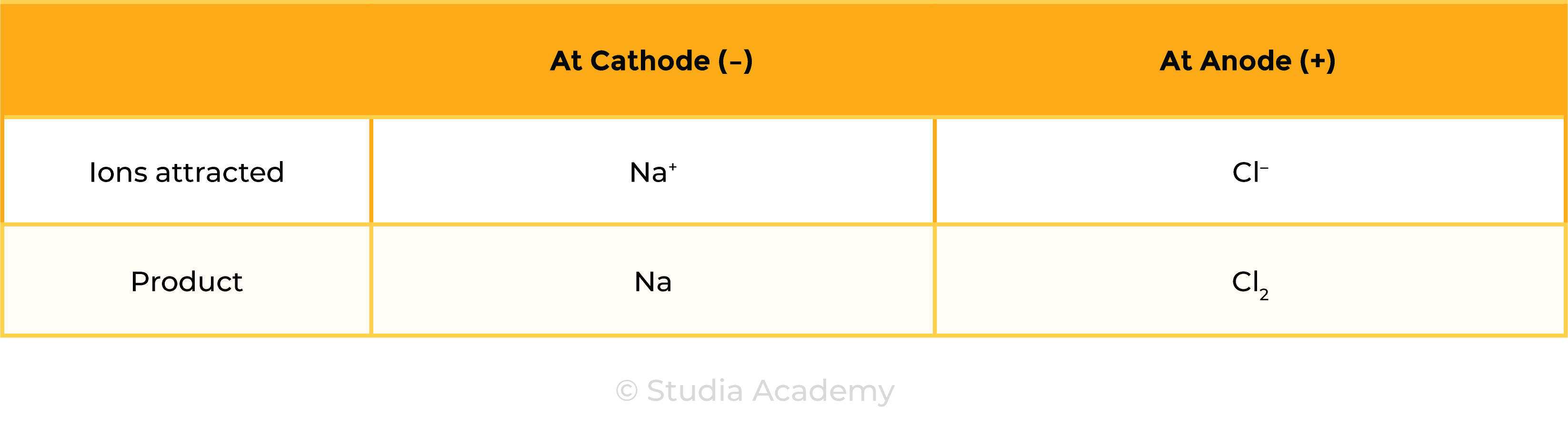
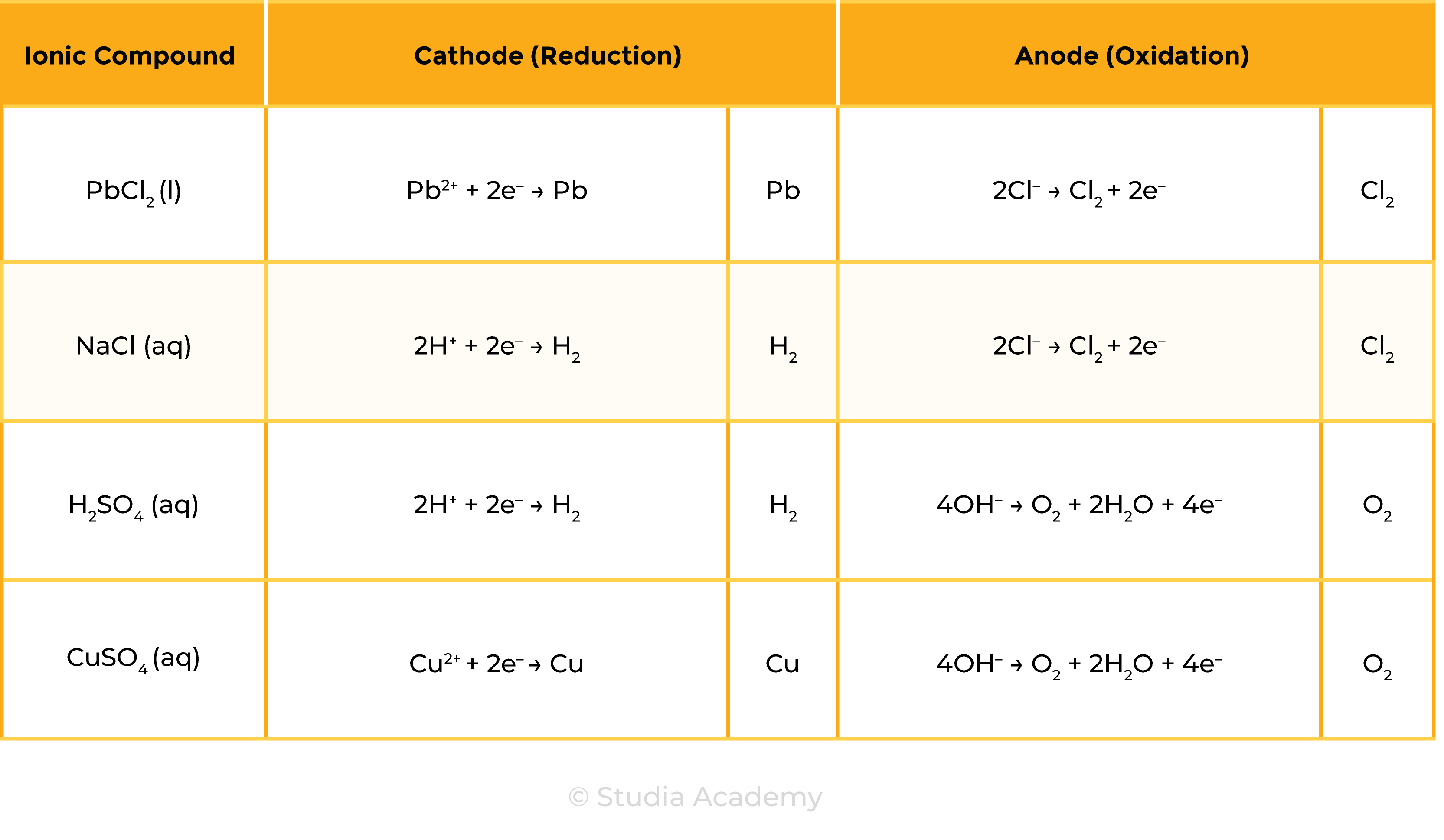
Example 1: Molten ionic compound, for example, NaCl (l)
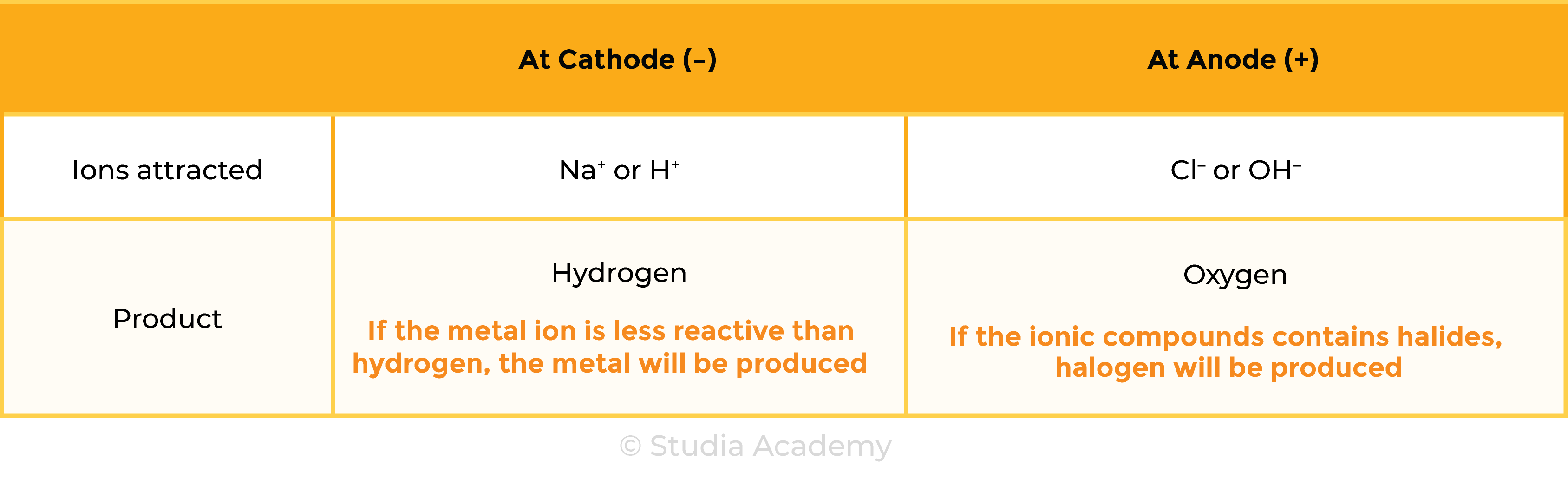
Example 2: Aqueous ionic compound, for example, NaCl (aq)
- Besides ions dissociated from ionic compounds, there are water molecules present
- In electrolytic solution: ions from ionic compounds, ions in water (OH– and H+)
Summary Table
1.9.5C Write ionic half-equations representing the reactions at the electrodes during electrolysis and understand why these reactions are classified as oxidation or reduction
Oxidation: loss of electron(s)
Reduction: gain of electron(s)
Key points
- Reduction takes place at cathode
- Reduction means electrons are gained
- So electrons should be on the left hand side of the half equation
- Oxidation takes place at anode
- Oxidation means electrons are lost
- So electrons should be on the right hand side of the half equation
1.9.6C Practical: investigate the electrolysis of aqueous solutions
AIM
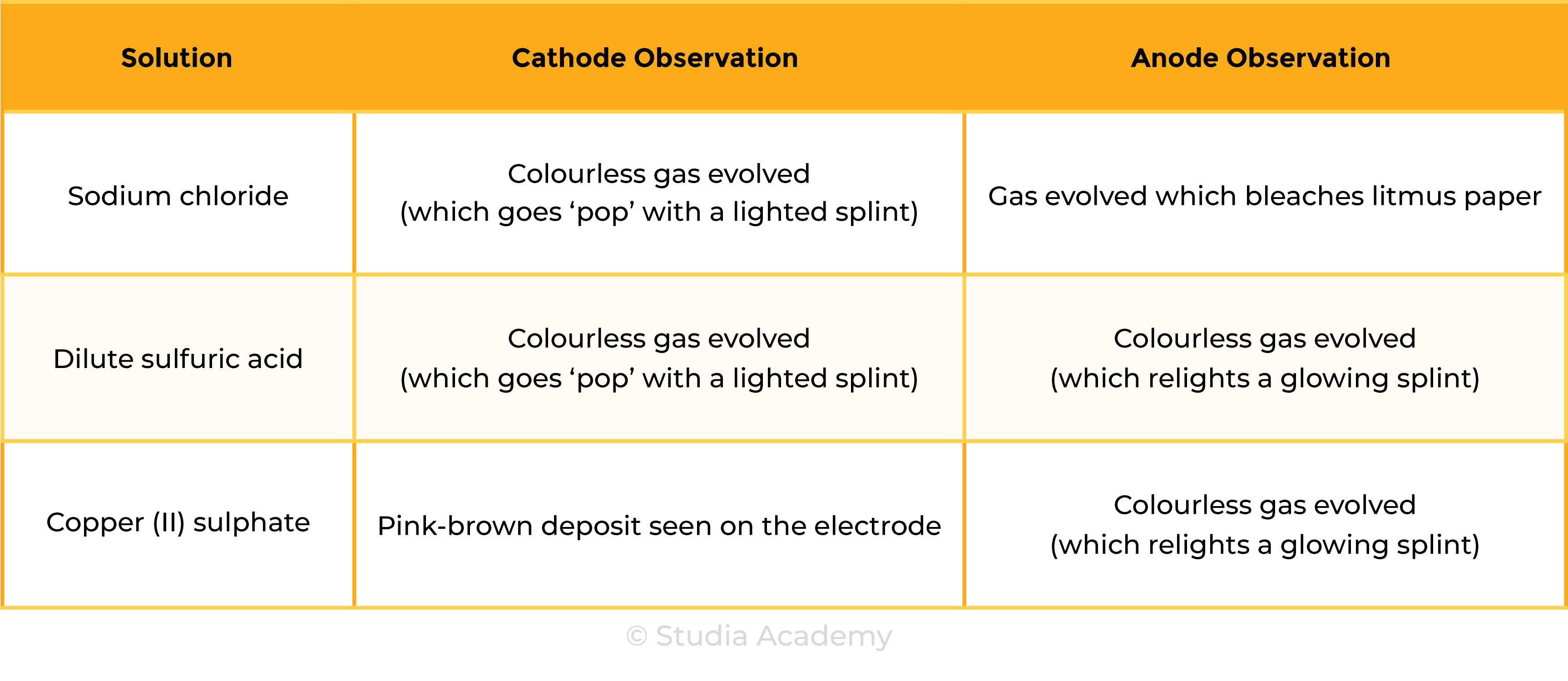
To electrolyse aqueous solutions of sodium chloride, sulfuric acid and copper(II) sulphate, and to collect and identify the products at each electrode
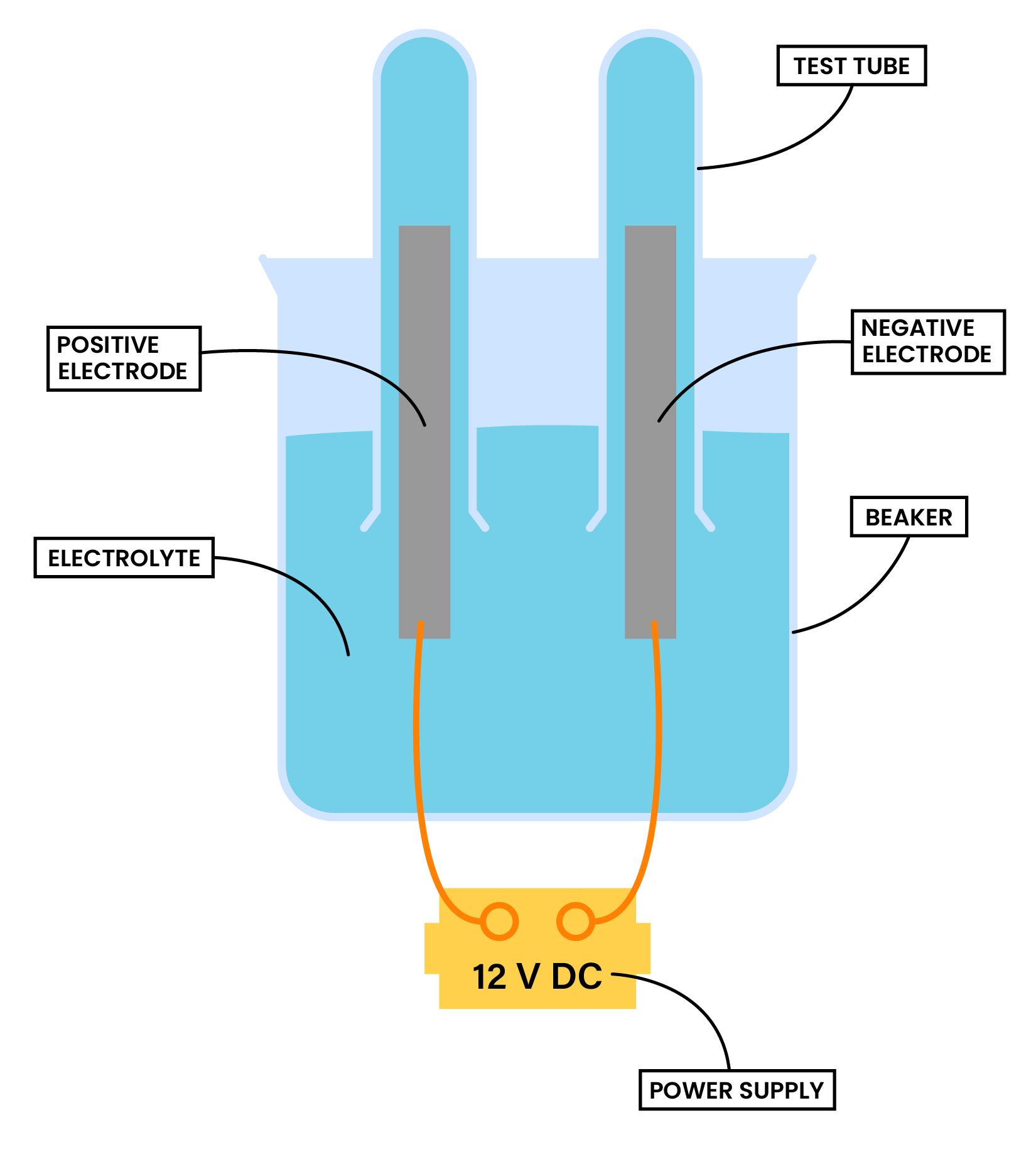
METHOD
- Add the aqueous solution to a beaker and cover the electrodes with the solution
- Invert two small test tubes to collect any gaseous products
- Connect the electrodes to a power supply.
- Turn on the power supply and allow electrolysis to take place
- Observations at each electrode are made
- Gases collected in the test tube can be tested and identified
Tests for Products

Results