REVISION NOTES
IGCSE Edexcel Chemistry
2.3 Gases in the Atmosphere
2.3.1 Know the approximate percentages by volume of the four most abundant gases in dry air
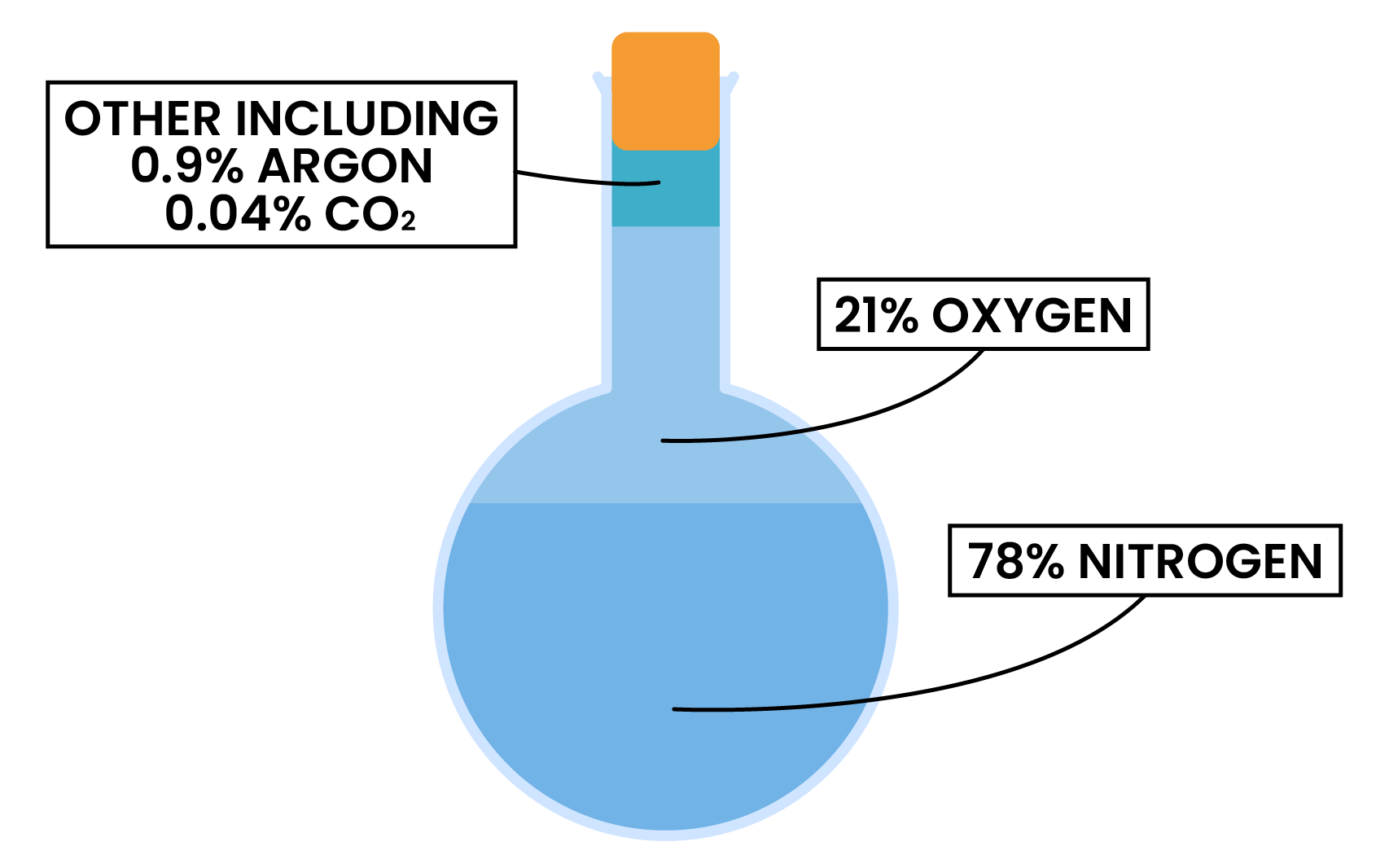
PROPORTION OF GASES
- The proportion of gases in the air has been relatively constant in the past 200 million years
- 78% nitrogen (N2)
- 21% oxygen (O2)
- 1% other gases (e.g. carbon dioxide CO2)
2.3.2 Understand how to determine the percentage by volume of oxygen in air using experiments involving the reactions of metals (e.g. iron) and nonmetals
(e.g. phosphorus) with air
The percentage of oxygen in the air can be found by:
- Reacting a metal (e.g. iron) in a fixed volume of air
- Reacting non-metal (e.g. phosphorus) in a fixed volume of air
- By calculating the volume of total air and the volume of combusted oxygen, the percentage of oxygen in the air can be found
Details of experiment will be discussed in 2.3.6
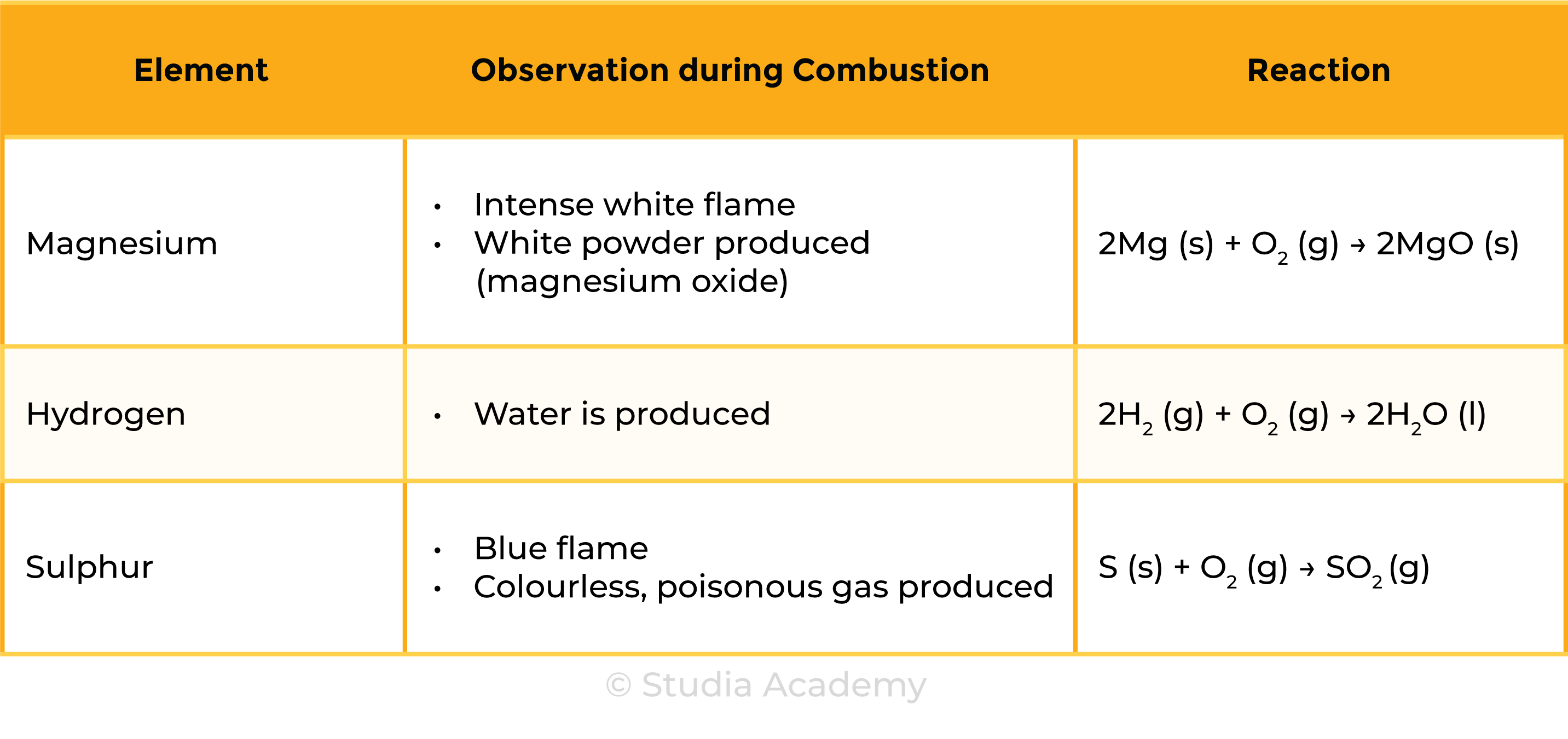
2.3.3 Describe the combustion of elements in oxygen, including magnesium, hydrogen and sulphur
COMBUSTION OF ELEMENTS
- Combustion is the process of burning
- All combustions involve chemical changes
- When elements react with oxygen, oxides are produced
- As there are new compounds formed, combustion is considered a chemical change
- All combustions release heat
- They are thus exothermic reactions
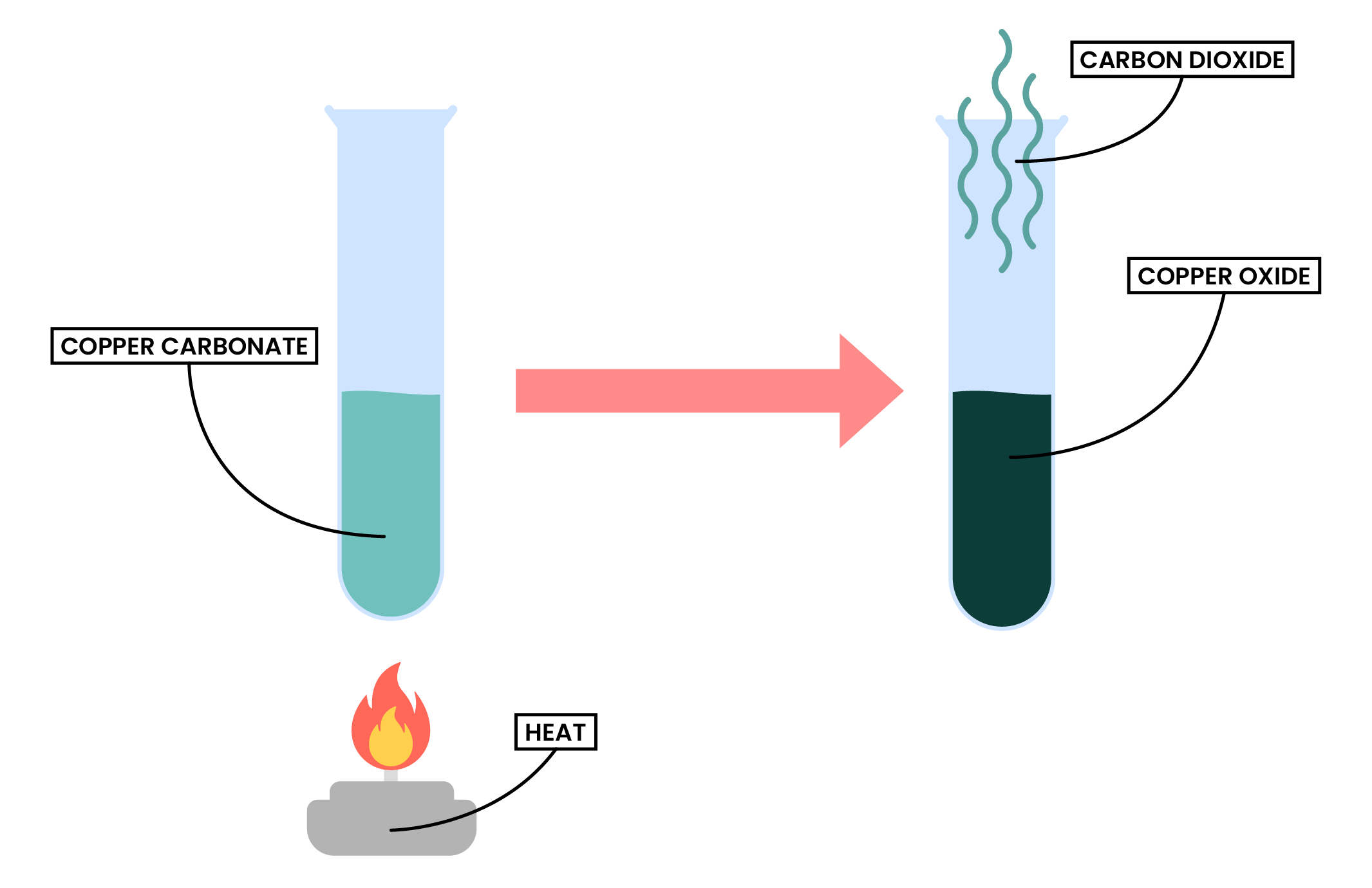
2.3.4 Describe the formation of carbon dioxide from the thermal decomposition of metal carbonates, including copper(II) carbonate
THERMAL DECOMPOSITION
- When a substance breaks down due to heat
- The compounds would decompose to several products
Thermal decomposition of metal carbonate
Metal carbonate → metal oxide + carbon dioxide
Example: copper (II) carbonate
CuCO3 (s) → CuO (s) + CO2 (g)
Copper (II) carbonate → Copper (II) oxide + Carbon dioxide
- Copper (II) carbonate is a green powder
- Copper (II) oxide produced is black
- Carbon dioxide produced can be tested by passing the gas through lime water and turning it milky
2.3.5 Know that carbon dioxide is a greenhouse gas and that increasing amounts in the atmosphere may contribute to climate change
ENHANCED GREENHOUSE EFFECT
- The Sun emits shortwave radiation to the Earth
- When the radiation hits the Earth’s surface, it is absorbed and re-emitted as infrared (IR) radiation
- However, the IR radiation is mostly trapped in the Earth’s atmosphere due to
greenhouse gases - Enhanced greenhouse effect: increasing levels of greenhouse gases trap extra heat energy in the Earth’s atmosphere
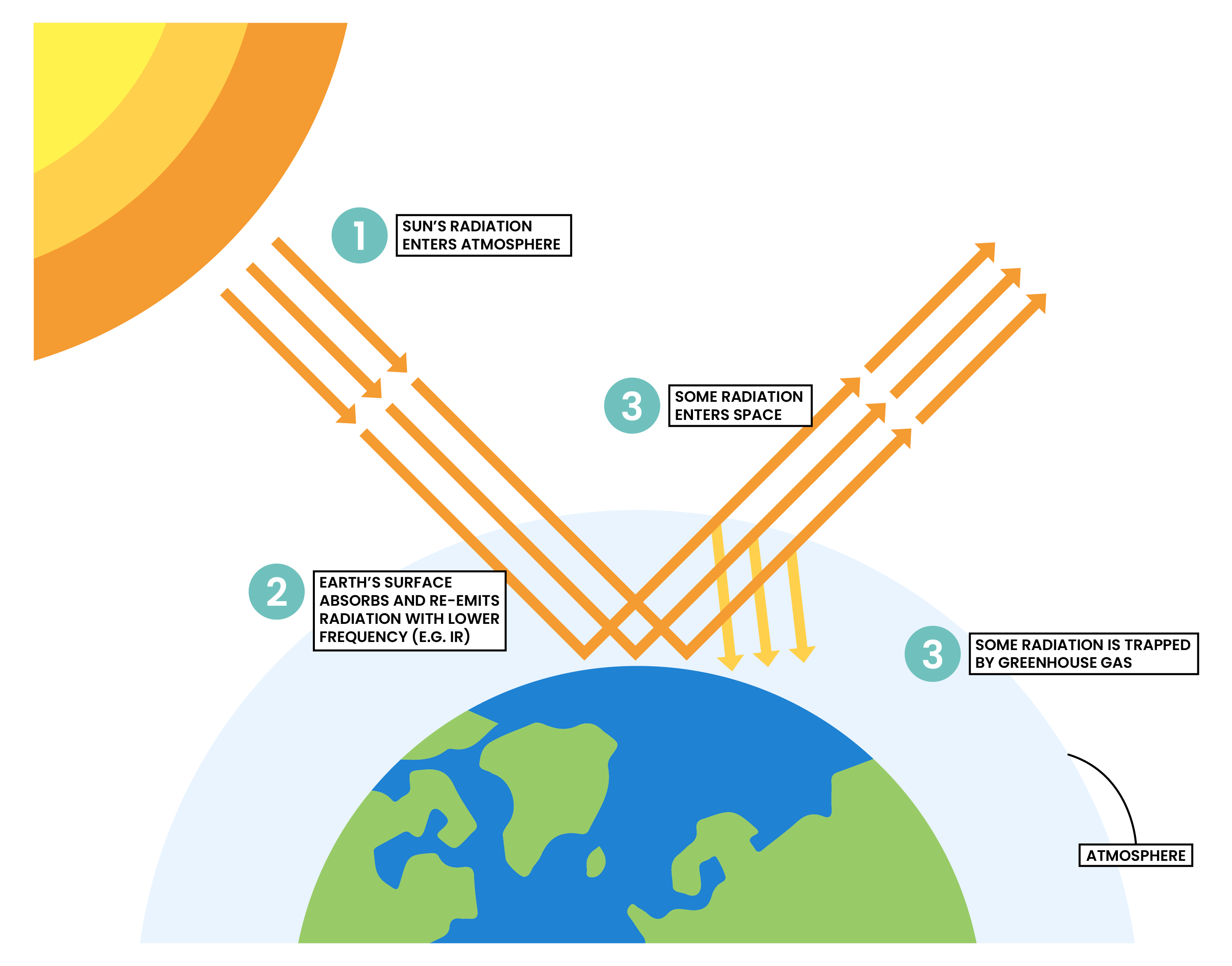
GREENHOUSE GASES
- Include carbon dioxide, methane and water vapour
- Sources of carbon dioxide:
- Combustion of wood and fossil fuels
- Respiration of plants and animals
- Thermal decomposition of carbonate rocks
- Effect of acids on carbonate
2.3.6 Practical: determine the approximate percentage by volume of oxygen in air using a metal or a non-metal
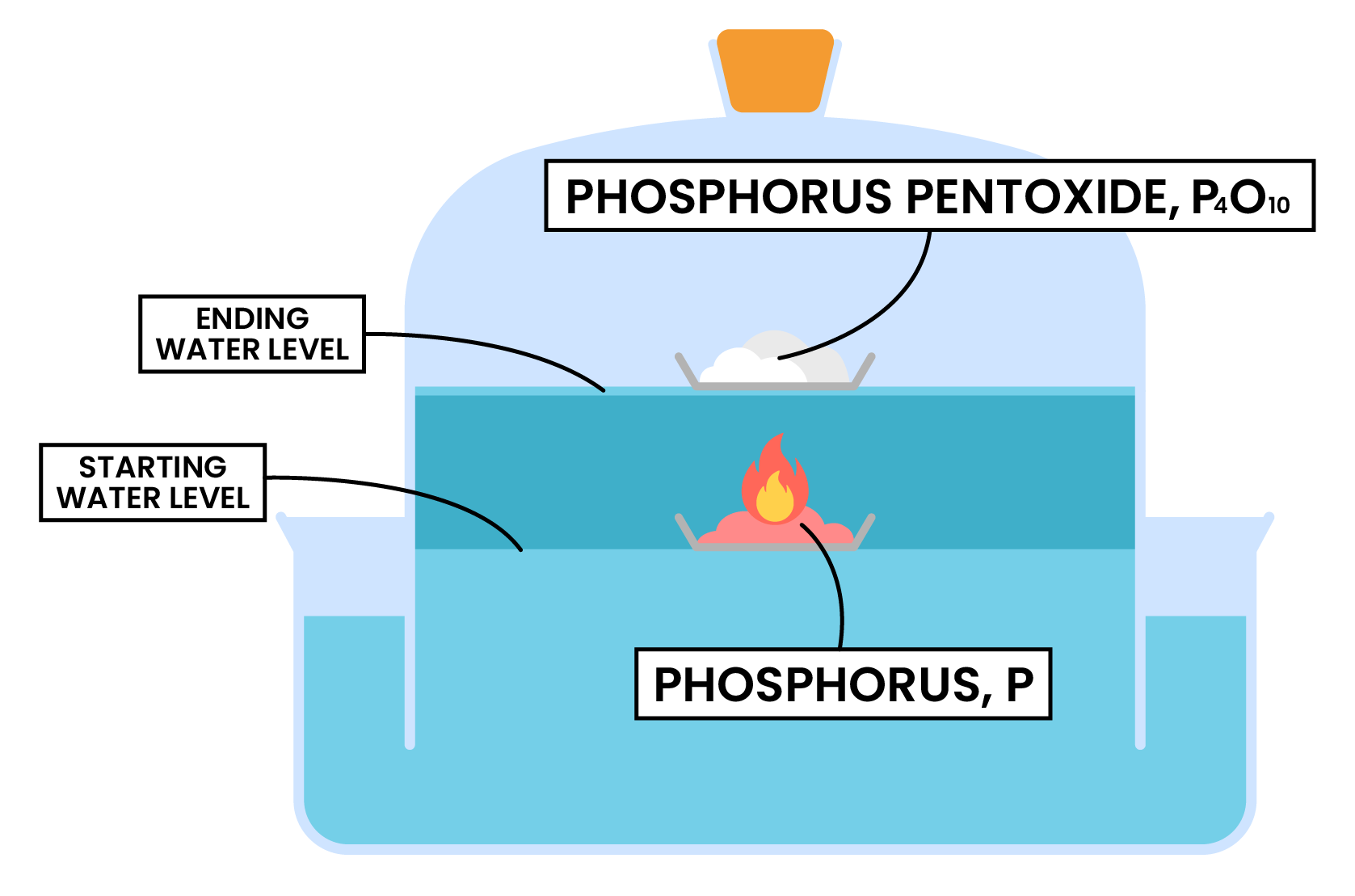
REACTION 1 COMBUSTION OF PHOSPHORUS
- Place a small amount of phosphorus in an evaporating dish
- Place the evaporating dish in a trough of water
- Burn the phosphorus in a bell jar
Measurement and calculations
- Initially the water levels inside and outside the jar should be the same
- As the phosphorus burns, oxygen inside the bell jar is used up
- The water level inside the bell jar rises
- By measuring the water levels before and after the combustion, percentage of oxygen can be calculated
Advantage and disadvantage
- Advantage: phosphorus is very suitable for this experiment as it burns readily until all oxygen available in the bell jar is used up
- Disadvantage: phosphorus is toxic, so it must be taken good care to handle it safely
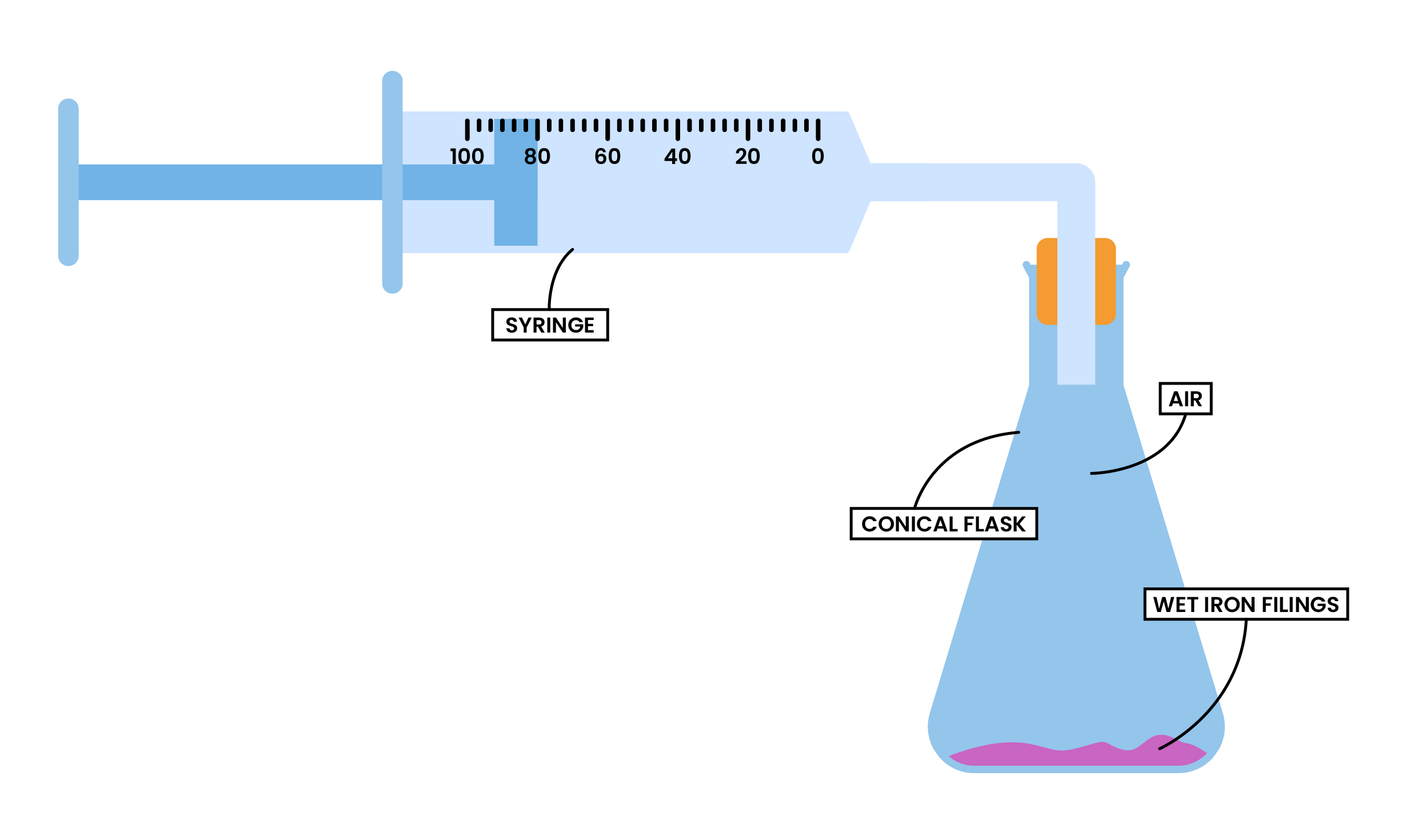
REACTION 2 OXIDATION OF IRON
- Iron reacts with oxygen in the air
- This process is called rusting (oxidation of iron)
- Water is needed for iron to rust too, so wet iron filings will be used
METHODS
- Place the wet iron filings in a conical flask
- Set up the experiment according to the diagram
- Measure and record the initial volume of gas
- After a few days, measure and record the final volume of gas
Calculation
- The final volume should be 4/5th of the initial volume
- The decrease in volume by about one-fifths shows that about 20% of the air is oxygen