REVISION NOTES
IGCSE Edexcel Chemistry
3.1 Energetics
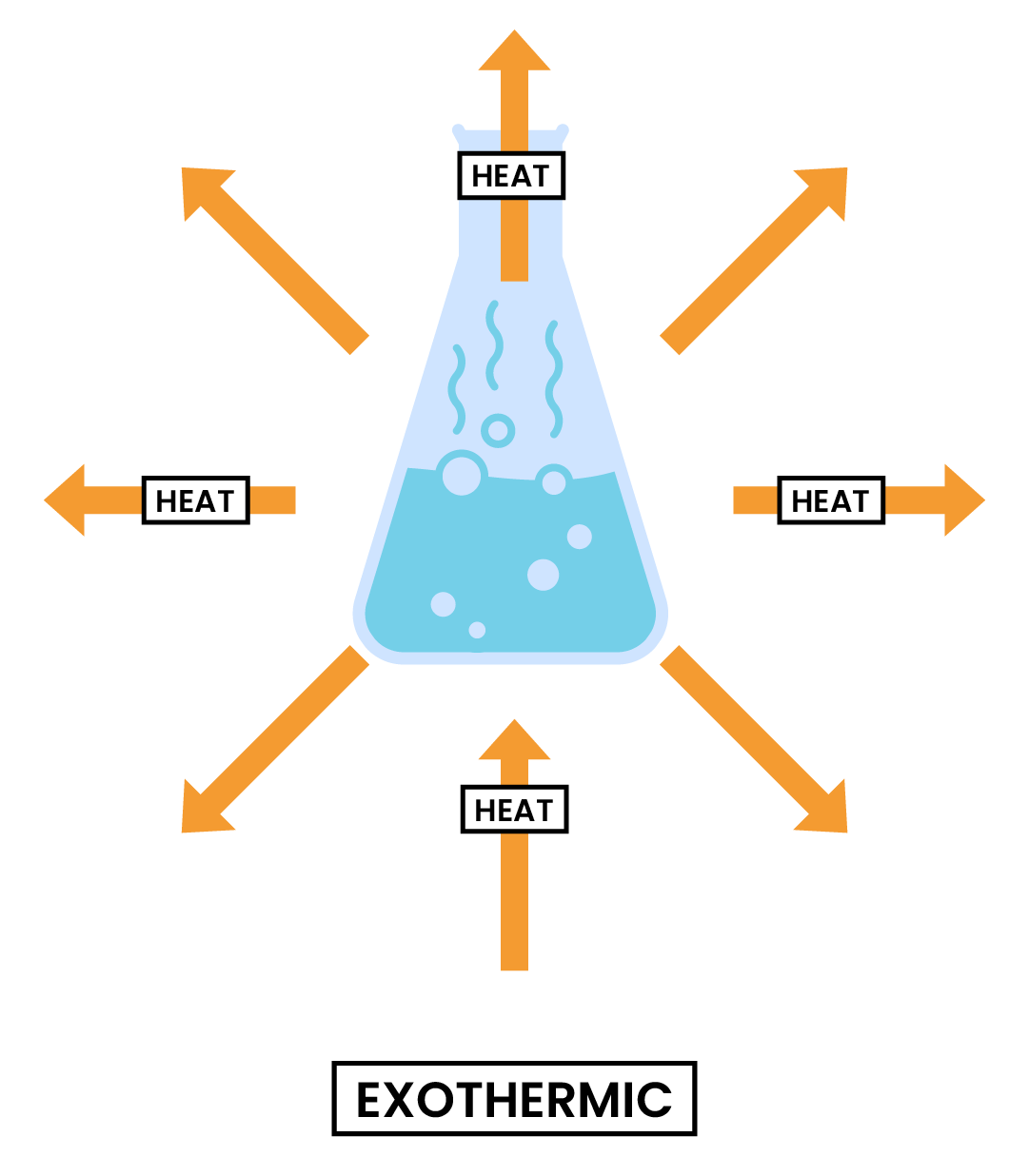
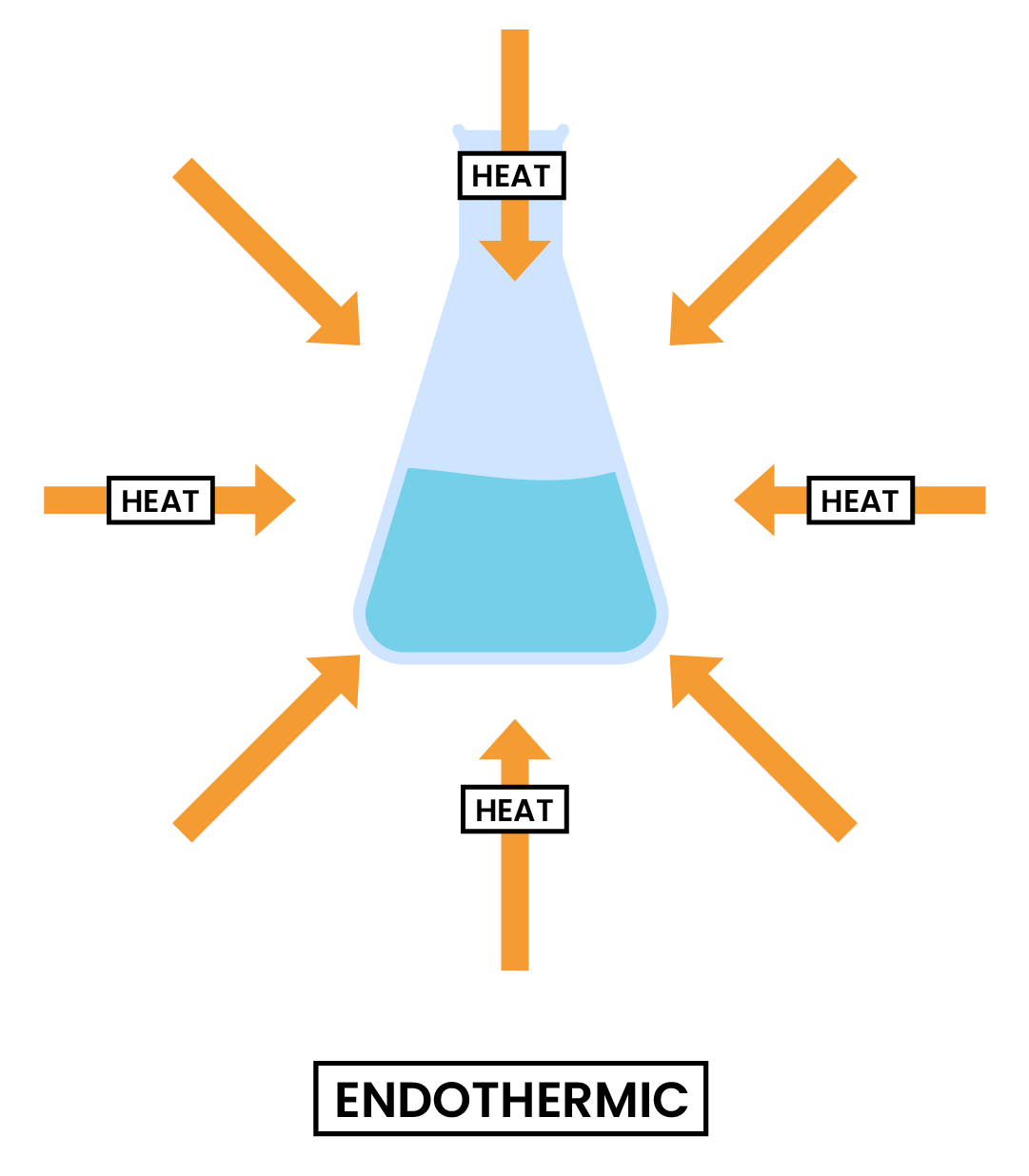
3.1.1 Know that chemical reactions in which heat energy is given out are described as exothermic, and those in which heat energy is taken in are described as endothermic

3.1.2 Describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation
CALORIMETRY
- A technique used to measure the enthalpy change of chemical reactions
- Enthalpy is a measure of heat content of a system
- There are two types of calorimetry experiments
- Reactions in solution
- Combustion
TYPE 1 REACTIONS IN SOLUTION
- Reactions include:
- Displacement
- Dissolving
- Neutralisation
- Assumptions are made about the experiments for calculations:
- Specific heat capacity of the solution is the same as pure water
- Density of the solution is the same as pure water
- Specific heat capacity of the container is ignored
- Reaction is complete
- There are negligible heat losses (to the surrounding)
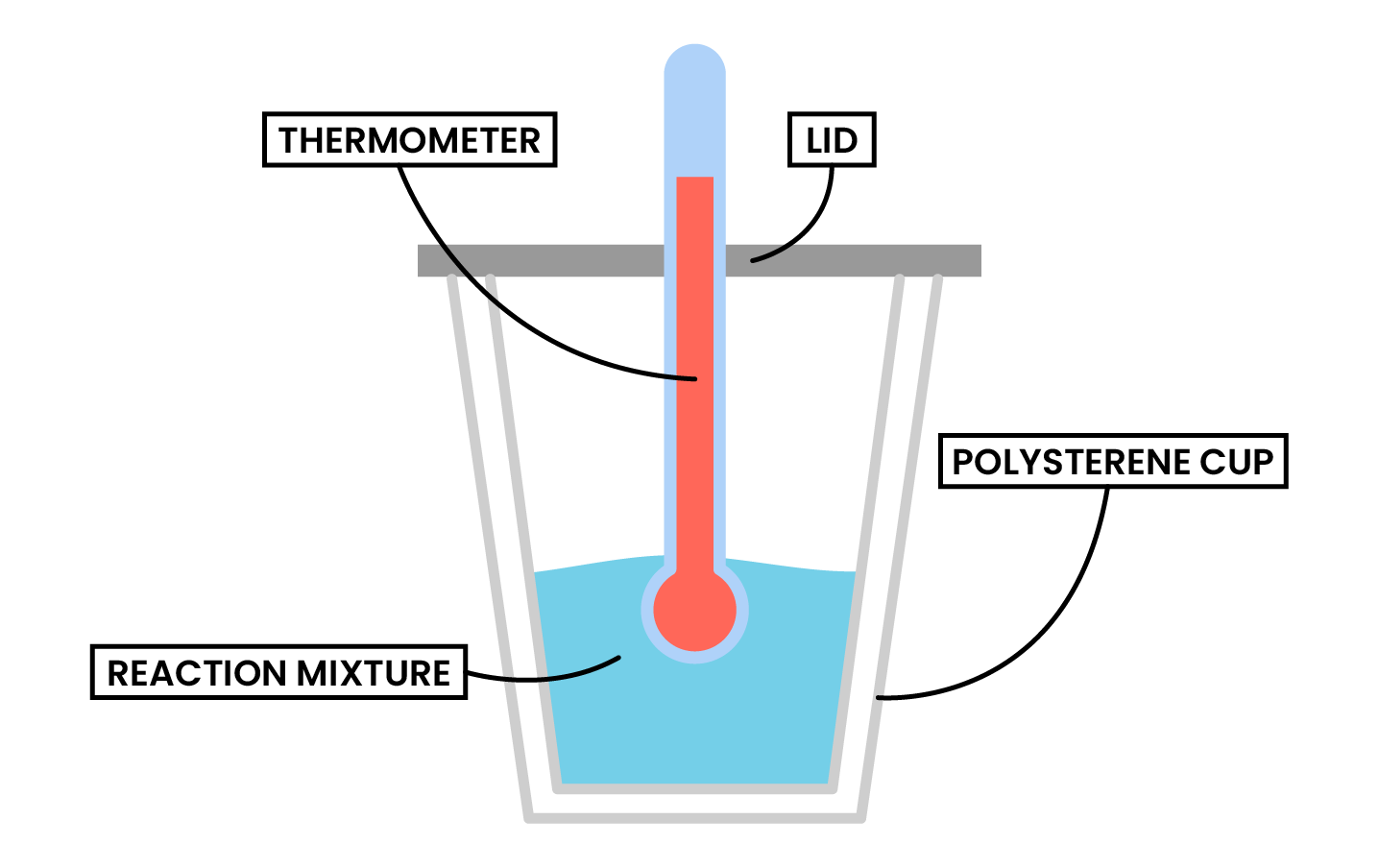
- A calorimeter can be made up of:
- Polystyrene drinking cup
- Vacuum flask
- Metal can
METHODS
- A fixed volume of one reagent is added to the calorimeter
- Measure and record the initial temperature
- An excess volume of another reagent is added
- The solution is stirred
- The maximum temperature is recorded and the temperature change is calculated
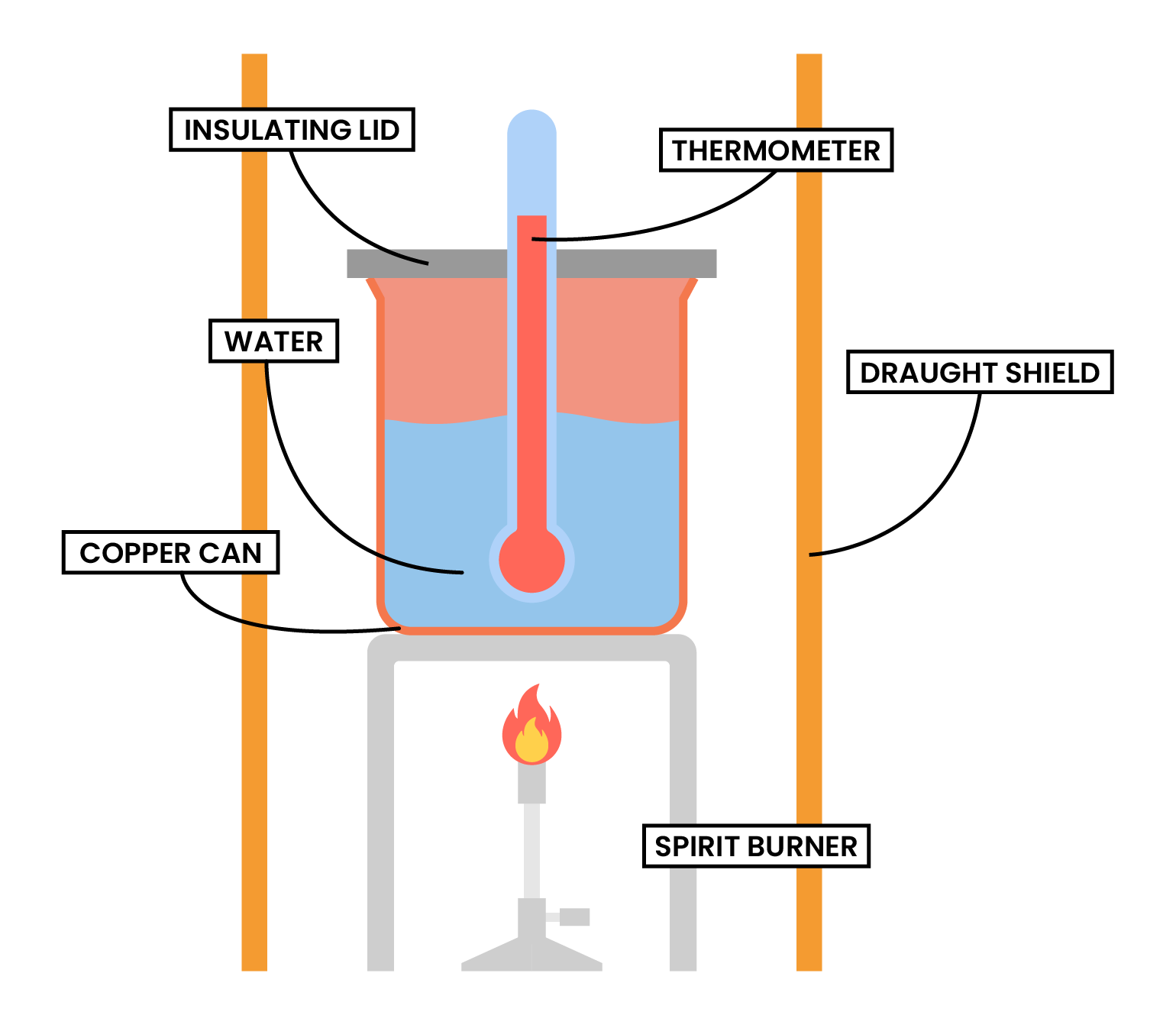
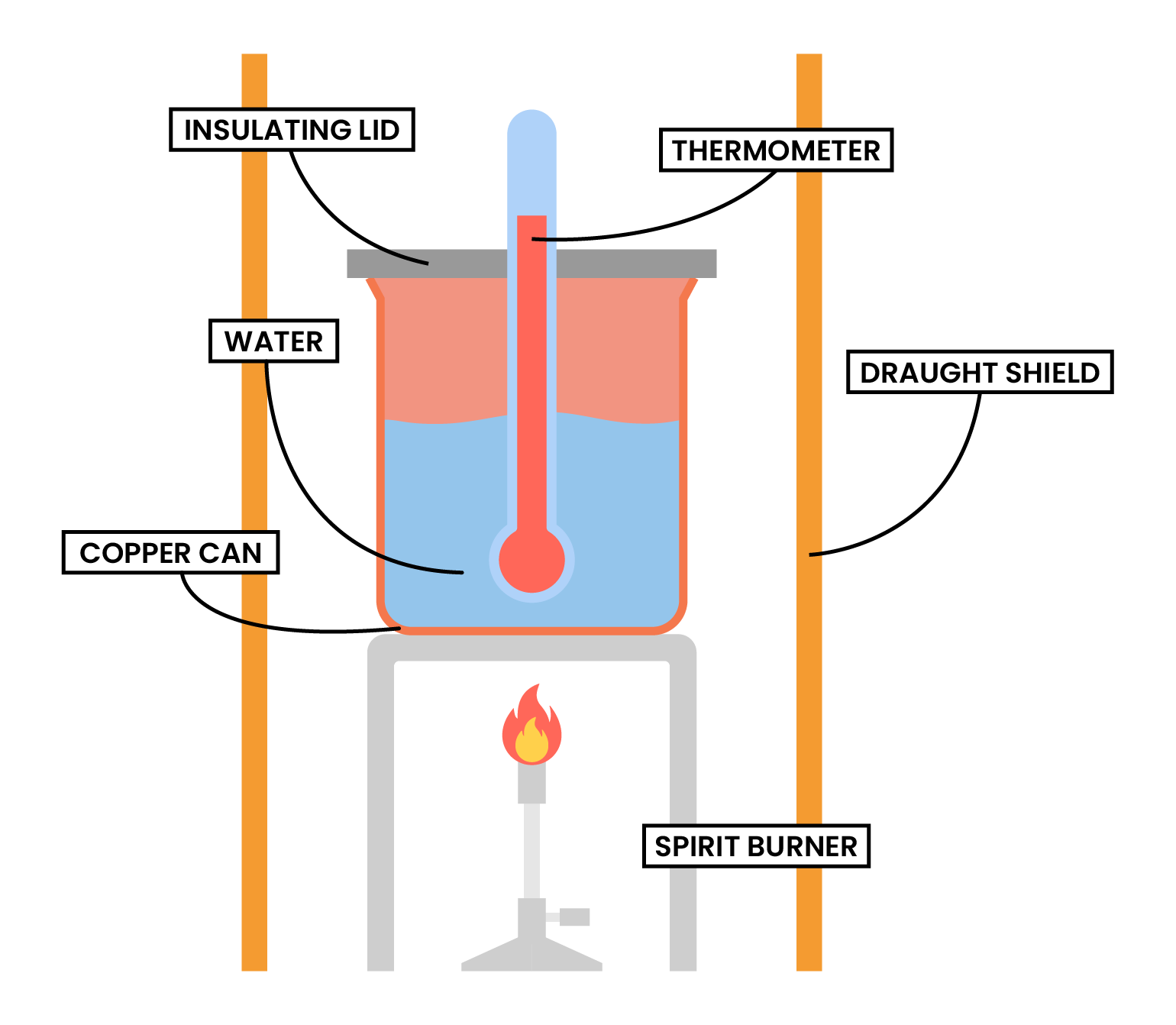
TYPE 2 COMBUSTION
- Heat released by the combustion reaction is used to increase the heat content of water
METHODS
- Measure and record the mass of the alcohol burner, together with the alcohol inside
- A fixed volume of water to added to a copper can
- Measure and record the initial temperature of the water
- Light the alcohol burner
- Stir the water continuously
- After a fixed period of time, put off the burner
- Measure and record the final temperature of the water
- Measure and record the mass of the alcohol burner
- Calculate the temperature change of the water
KEY POINTS
- Instead of an alcohol burner, any combustion (e.g. combustion of food) can be used to increase the temperature of water
- However, there is heat loss during the process
- Not all the heat produced by the combustion reaction is used to heat the water
- Some heat is lost to the surroundings
- Some heat is absorbed by the calorimeter
- To minimise the heat loss:
- The calorimeter should not be placed too far above the flame
- A lid should be placed over the calorimeter
- Draught shield can be used
- In this experiment the main sources of error are
- Heat losses
- Incomplete combustion
3.1.3 Calculate the heat energy change from a measured temperature change using the expression Q = mcΔT
SPECIFIC HEAT CAPACITY (c)
SPECIFIC HEAT CAPACITY (c)
- Definition: energy needed to raise the temperature of 1g of a substance by 1K
- The specific heat capacity of water is 4.18 J g–1 K–1
Energy transferred as heat can be calculated by:
q = m ✕ c ✕ ΔT
q = heat transferred (J)
m = mass of water (g)
c = specific heat capacity (J g–1 K–1)
ΔT = temperature change (K)
KEY POINTS
- Temperature change in Kelvin is the same as the temperature change in degrees Celsius
- Mass of water should be included, not the mass of alcohol (or other compounds) that undergoes combustion
- The energy calculated is in Joules (J)
ΔT = final temperature – initial temperature
= 58oC – 24oC
= 34oC
q = m ✕ c ✕ ΔT
= 300 ✕ 4.18 ✕ 34
= 42636 J
= 42.636 kJ
3.1.4 Calculate the molar enthalpy change (ΔH) from the heat energy change, Q
MOLAR ENTHALPY CHANGE
- Heat energy change per mole of substance
- Symbol: ΔH
- Unit: kJ per mole
CALCULATION OF ΔH [TITLE]
- First, determine the heat energy change of the reaction, q, using q = m × c × ΔT
- Then, divide q by the number of moles, n, of the substance
- Remember the negative sign
- Heat released by the reaction is absorbed by the water, increasing the temperature
Molar enthalpy change = heat change for the reaction ÷ number of moles
ΔH = – q ÷ n
EXAMPLE (CONT.)
2.087 g of ethanol (Mr = 46) was burned in an alcohol burner and used to heat 300 g of water in a calorimeter. The initial and final temperature of water was 24oC and 58oC respectively. Calculate the molar enthalpy of combustion.
q = m ✕ c ✕ ΔT
= 42.636 kJ
(from 3.3)
n = mass (ethanol) ÷ molar mass (ethanol)
= 2.087 ÷ 46
= 0.0454 mol
ΔH = – q ÷ n
= – 42.636 ÷ 0.0454
= – 939 kJ per mol
3.1.5C Draw and explain energy level diagrams to represent exothermic and endothermic reactions
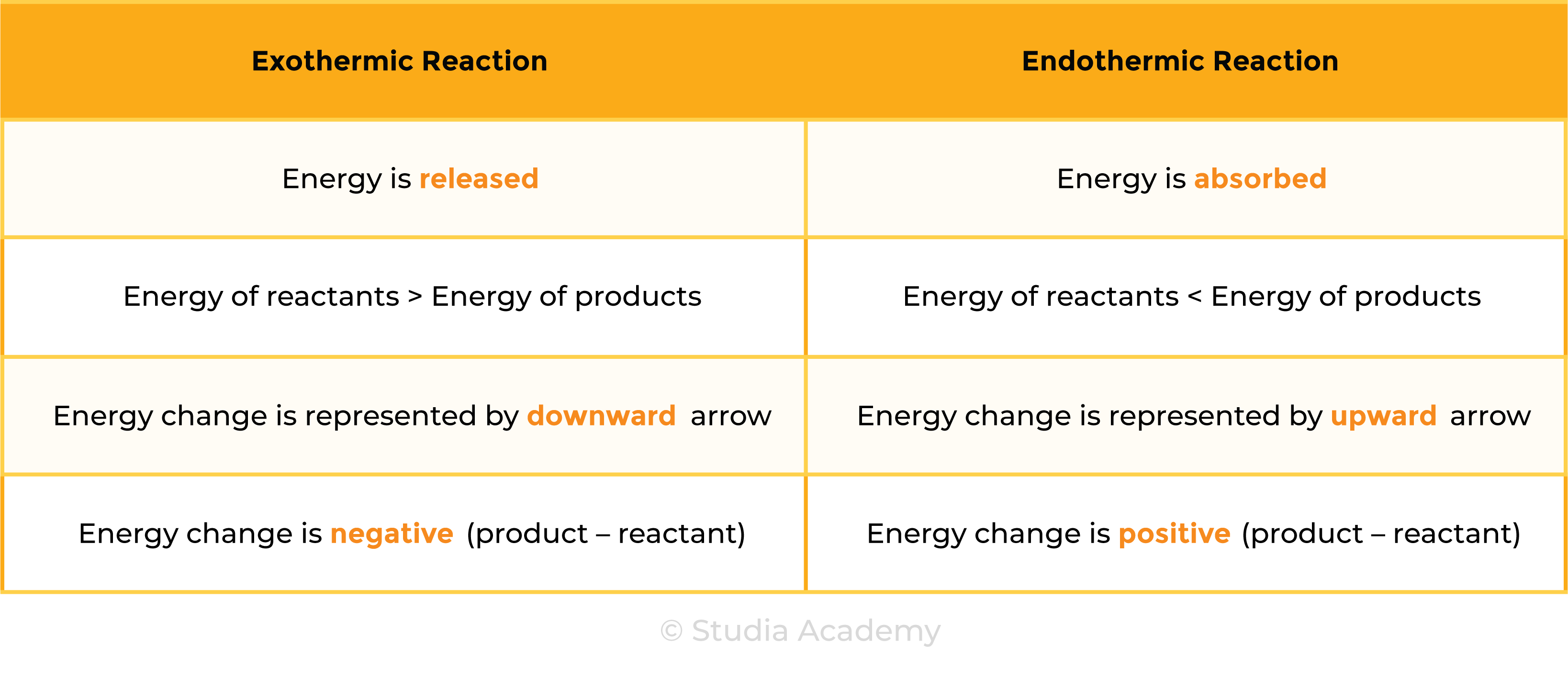
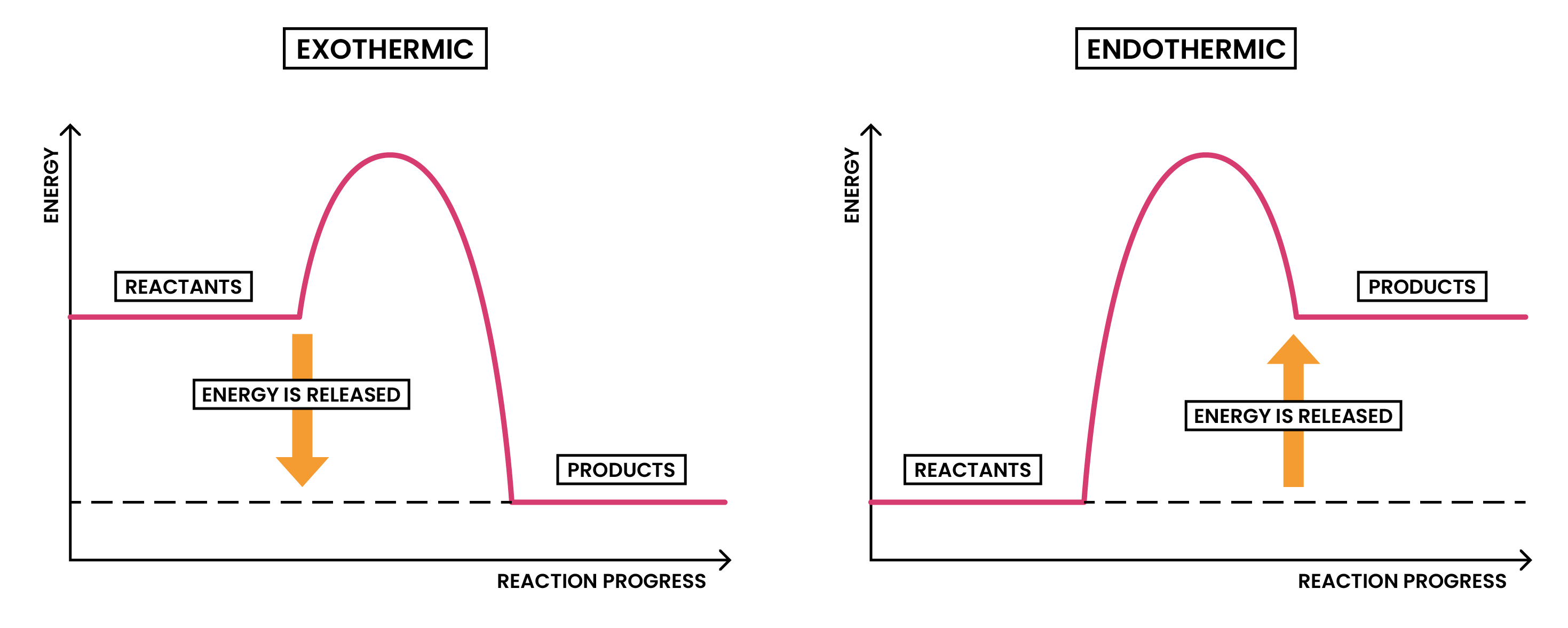
ENERGY LEVEL DIAGRAMS
- Graphical representations of energies of reactants and products in chemical reactions
- x-axis: reaction pathway (reaction progress)
- y-axis: energy
- Overall energy change: difference between energies of reactants and products
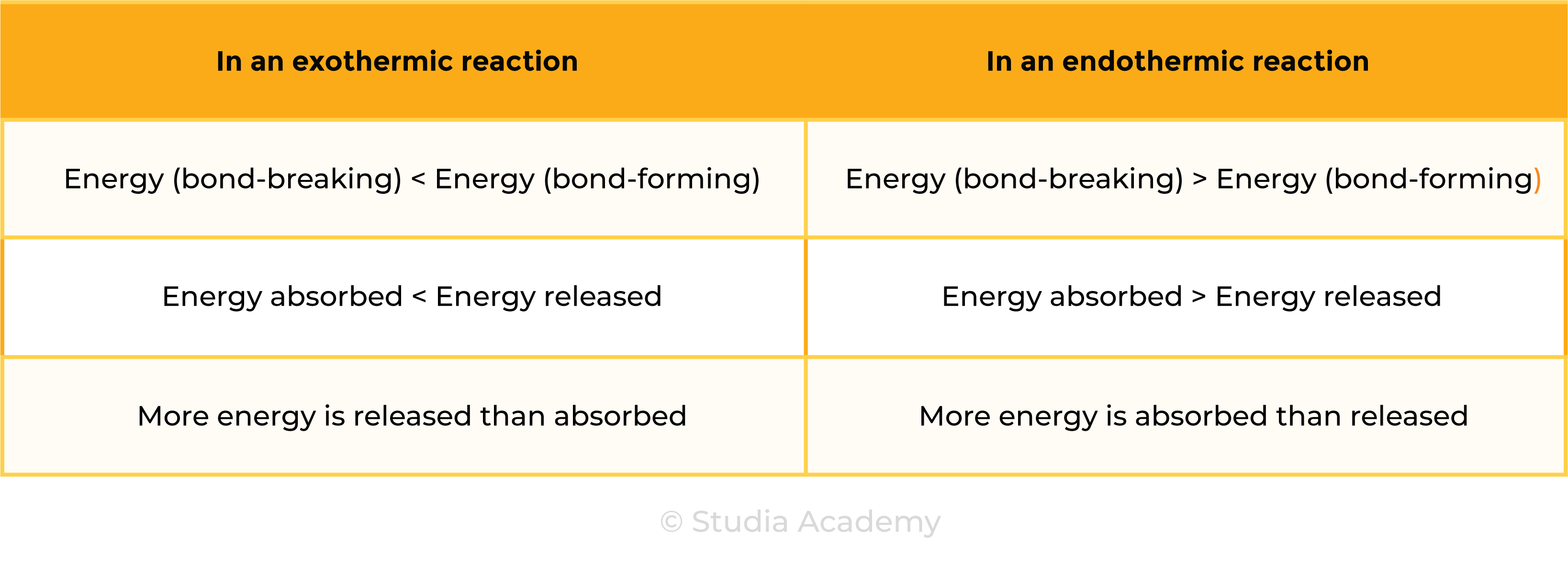
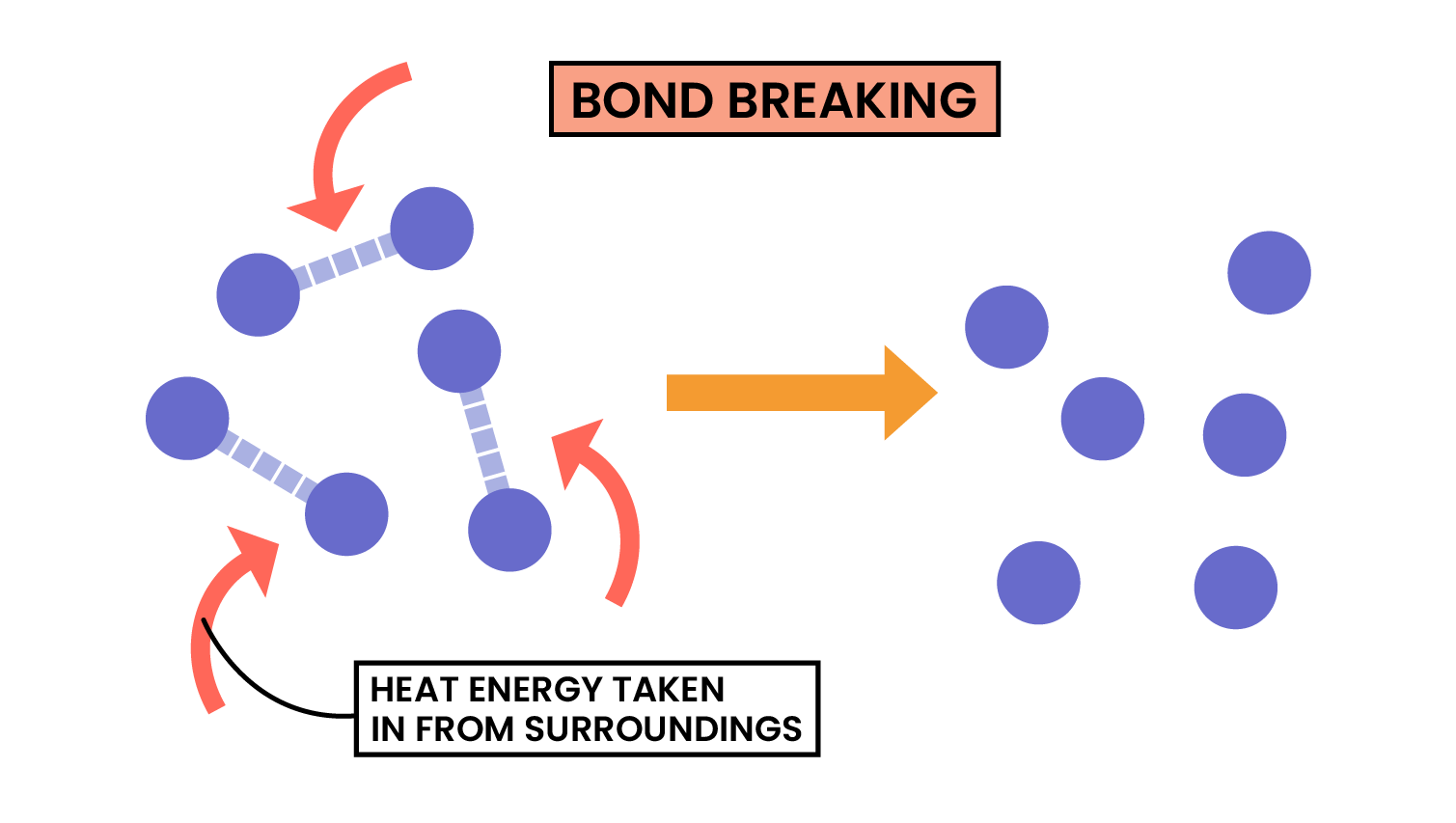
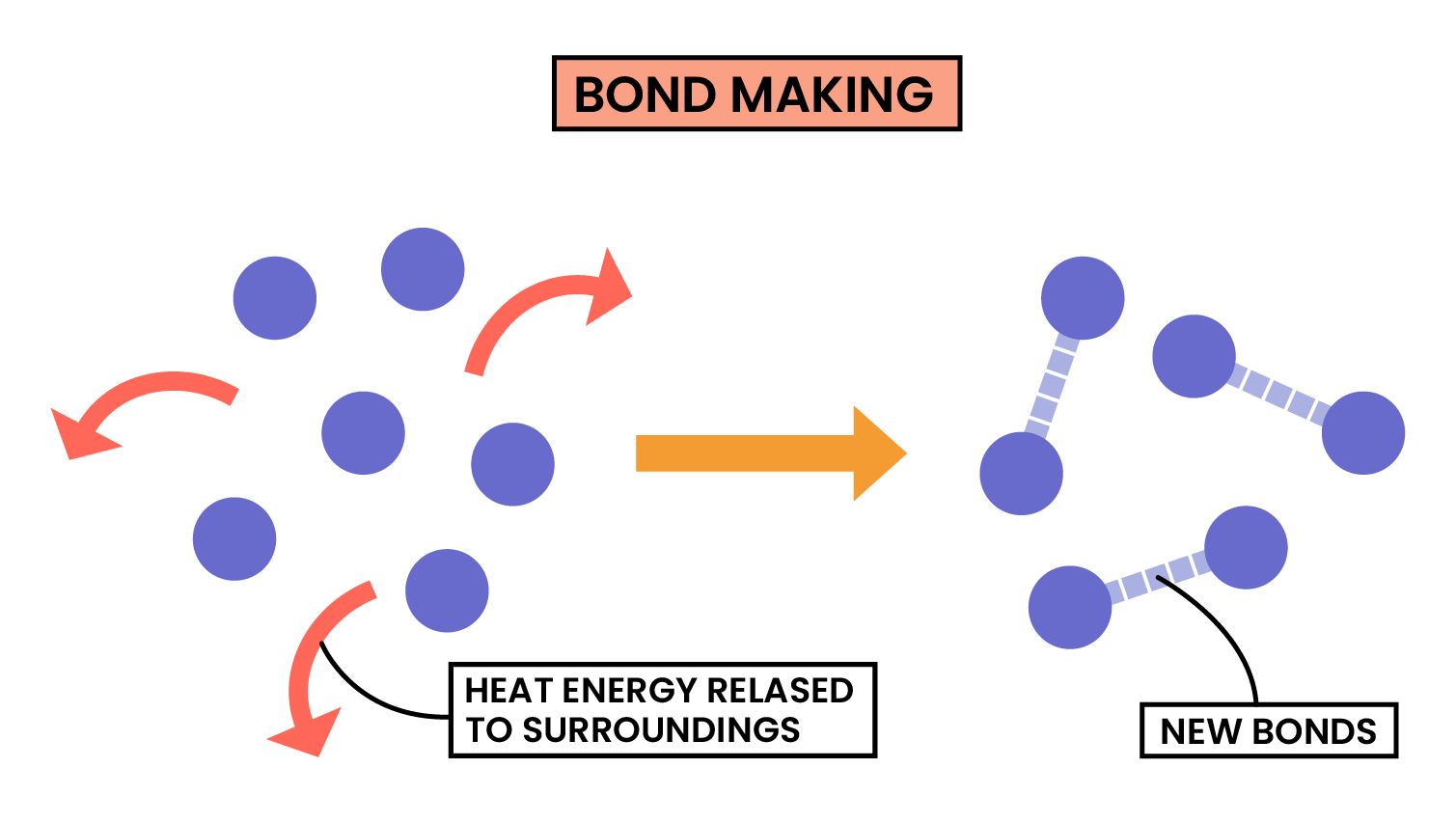
3.1.6C Know that bond-breaking is an endothermic process and that bond-making is an exothermic process
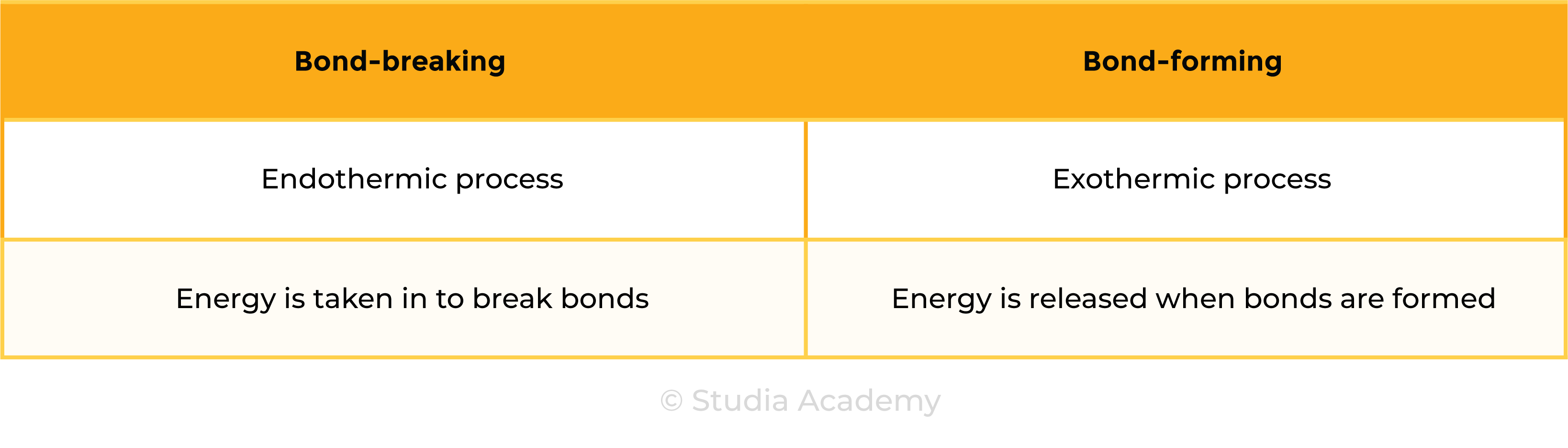
Use the idea of magnets:
Pulling apart magnets or bond breaking, requires energy, so is endothermic.
Pushing magnets together or bond making, does not require energy (releases energy in bond making), so is exothermic.
3.1.7C Use bond energies to calculate the enthalpy change during a chemical reaction
ENTHALPY CHANGE
- Enthalpy change can be calculated by using bond energies of reactants and products
Enthalpy change = Energy taken in – Energy given out
Enthalpy change = Bond Energies of Reactants – Bond Energies of Products
EXAMPLE
Calculate the enthalpy change for the following reaction, using the bond energies given:
CH4 + 2O2 → CO2 + 2H2O
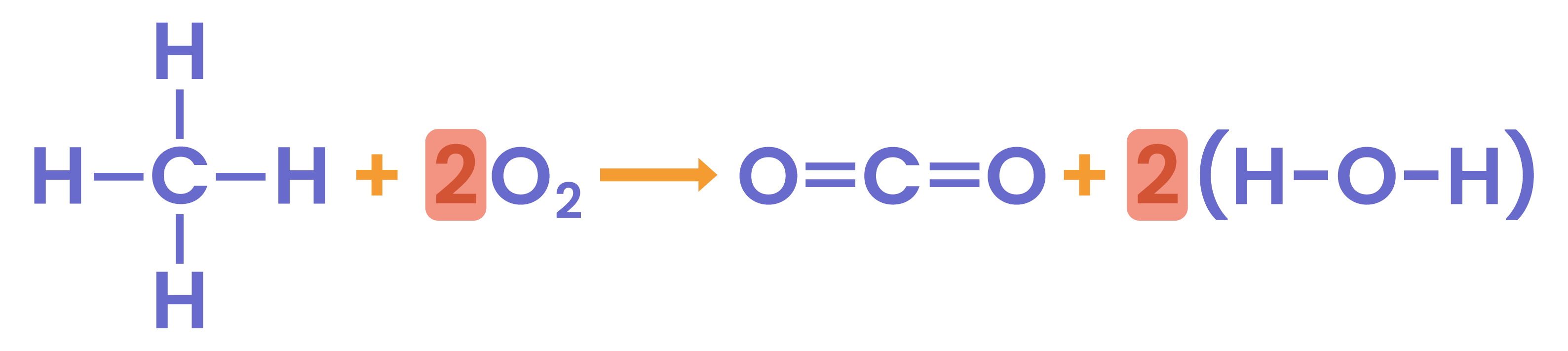
Bond energies of reactants
= 4 ✕ (C – H) + 2 ✕ (O = O)
= 4 ✕ 413 + 2 ✕ 495
= 2642 kJ
Bond energies of products
= 2 ✕ (C = O) + 4 ✕ (O – H)
= 2 ✕ 799 + 4 ✕ 463
= 3450 kJ
Enthalpy change
= bond energies of reactants – bond energies of products
= 2642 – 3450
= – 808 kJ/mol
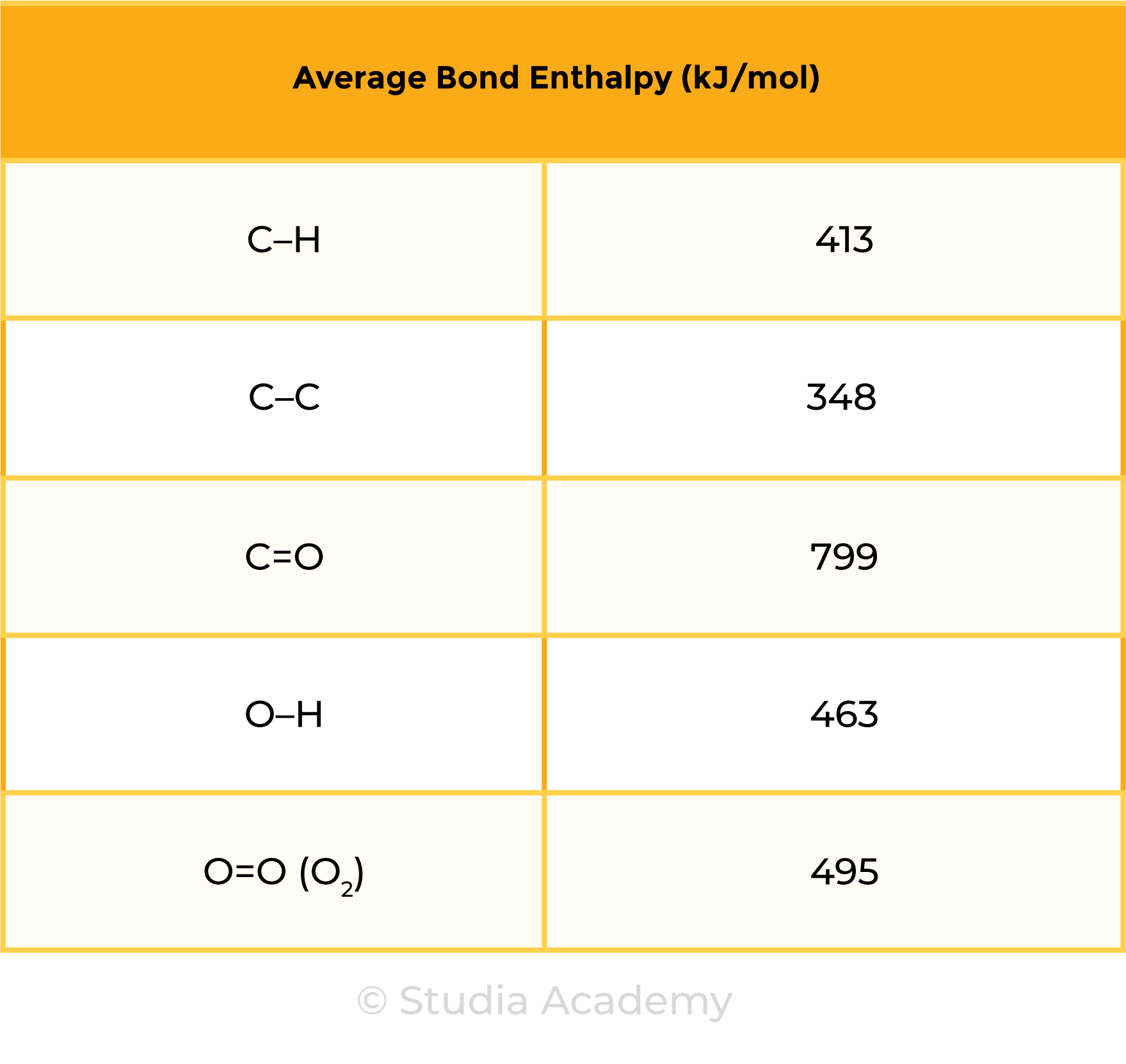
3.1.8 Practical: investigate temperature changes accompanying some of the following types of change:
- Salts dissolving in water
- Neutralisation reactions
- Displacement reactions
- Combustion reactions.
Type 1 Calorimetry Experiment (Dissolving, Neutralisation and Displacement)
METHODS
- Use a measuring cylinder to measure 25 cm3 of solution 1 into a polystyrene cup
- Measure and record the initial temperature
- Add a measured mass/volume of reactant into the polystyrene cup
- Solid for dissolving
- Solution 2 for neutralisation
- Solution 2 for displacement
- Stir the mixture
- Measure and record the highest temperature reached by the reaction as final temperature
- Calculate the temperature change = final temperature – initial temperature
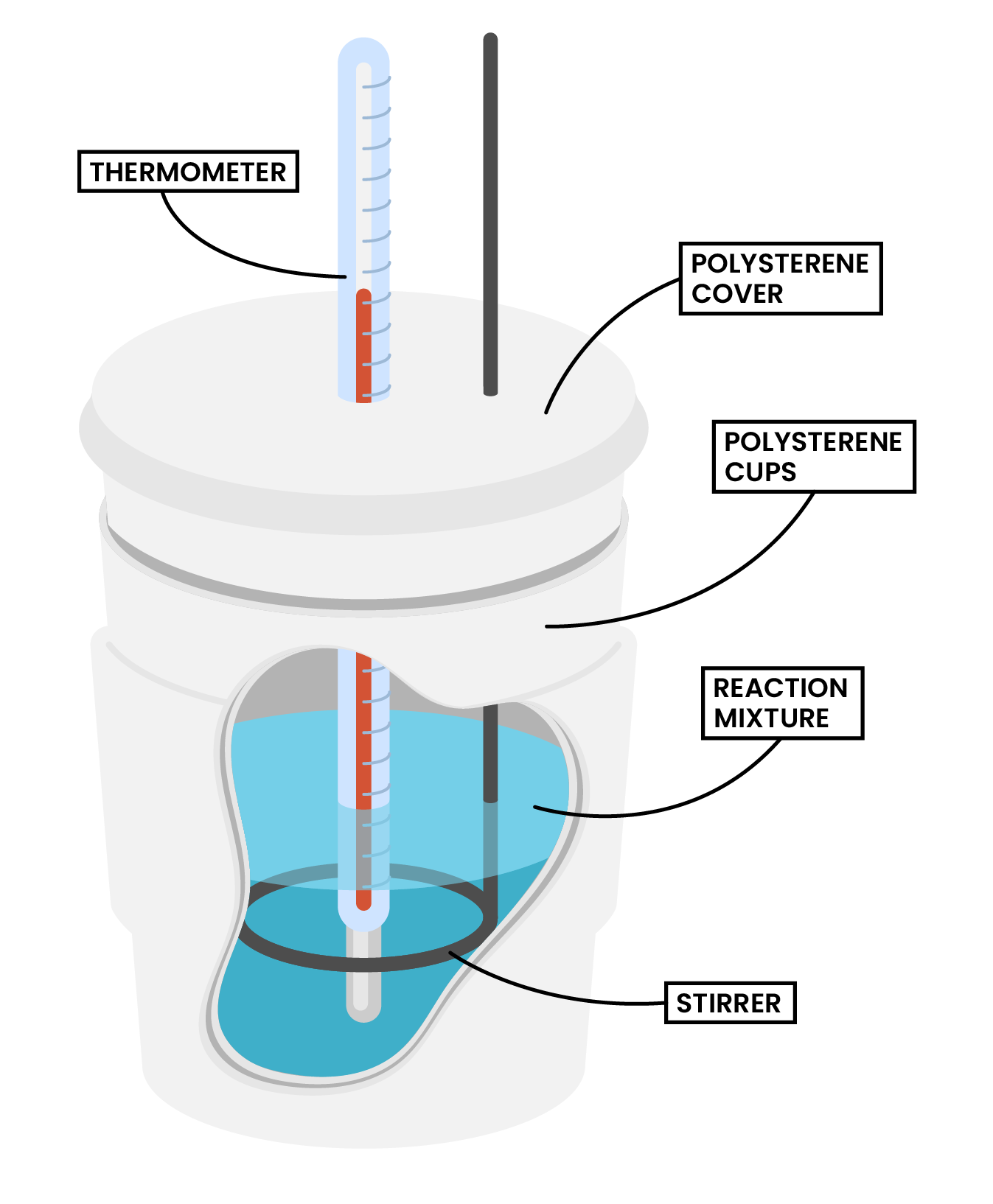
Type 2 Calorimetry Experiment (Combustion)
METHODS
- Use a measuring cylinder to measure 100 cm3 of water into a copper can
- Measure and record the initial temperature
- Fill the spirit burner with substance to be combusted
- Measure and record the initial mass of spirit burner + substance
- Place the spirit burner under the copper can and light the wick
- Constantly stir the water
- After a fixed period of time, put off the burner
- Measure and record the final temperature of the water
- Measure and record the final mass of the spirit burner
- Calculate the temperature change of the water = final temperature – initial temperature