REVISION NOTES
IGCSE Edexcel Chemistry
3.2 Rates of Reaction
3.2.1 Describe experiments to investigate the effects of changes in surface area of a solid, concentration of a solution, temperature and the use of a catalyst on the rate of a reaction
In combination with 3.2.7 and 3.2.8
Investigate:
- Effect of surface area of a solid on rate of reaction
- Effect of concentration of a solution on rate of reaction
- Effect of temperature on rate of reaction
- Effect of the use of a catalyst on rate of reaction
Key Points
- When investigating factors affecting rate of reactions, dependent variable is rate of reaction (speed of reaction)
- Mass / Volume of products is measured over a period of time
- Rate of reaction = Change in mass/volume of products ÷ Change in time
Factor 1 Surface Area of A Solid
calcium carbonate + hydrochloric acid → calcium chloride + carbon dioxide + water
CaCO3(s) + 2HCl(aq) → CaCl2(s) + CO2(g) + H2O(l)
- As carbon dioxide is produced, the gas can be collected and the volume can be measured
- Independent variable: surface area of calcium carbonate
- Dependent variable: volume of carbon dioxide collected in a fixed time
- Expected result:
- The larger the surface area (smaller particles), the larger the volume of carbon dioxide collected, the higher the rate of reaction
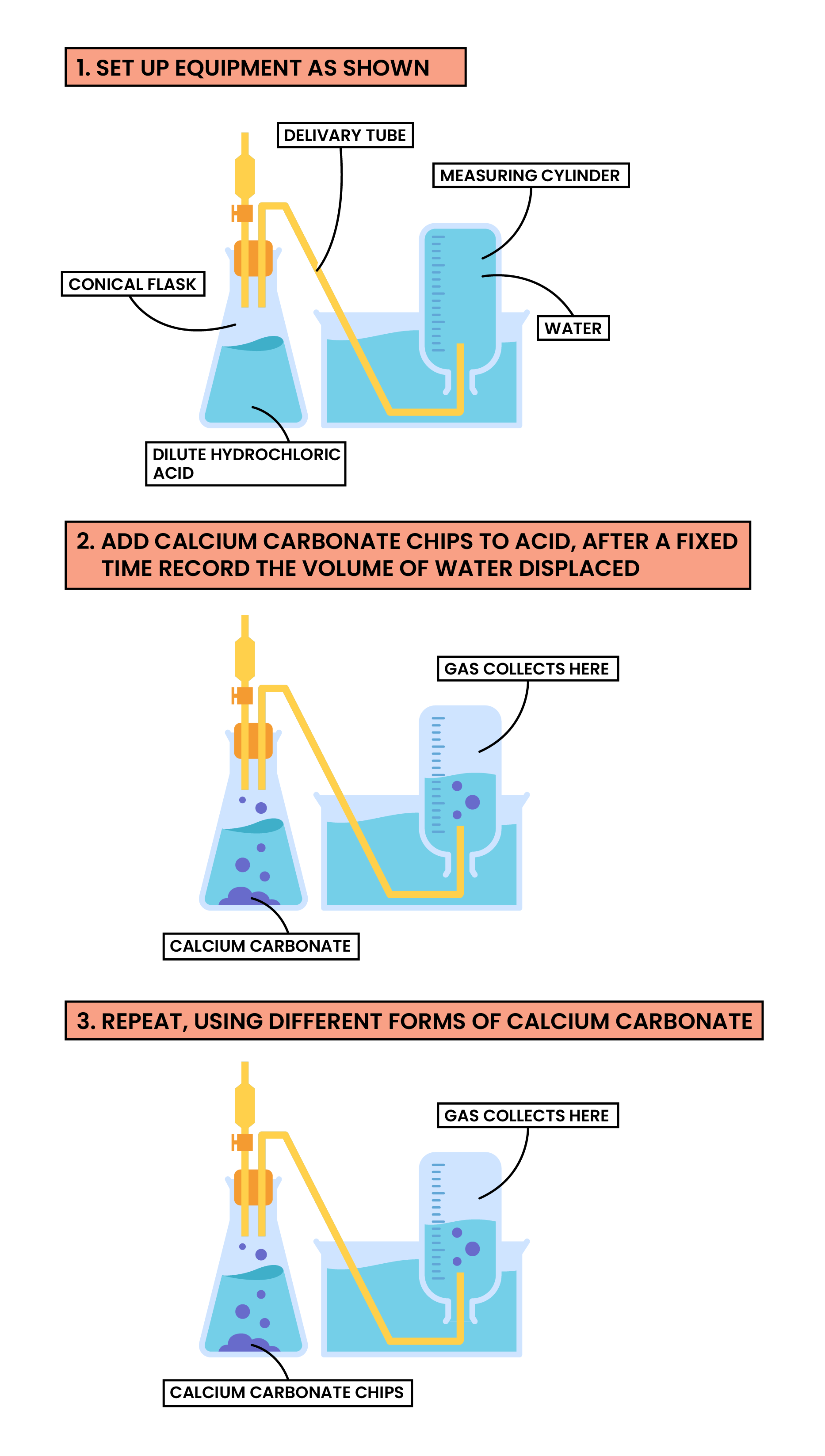
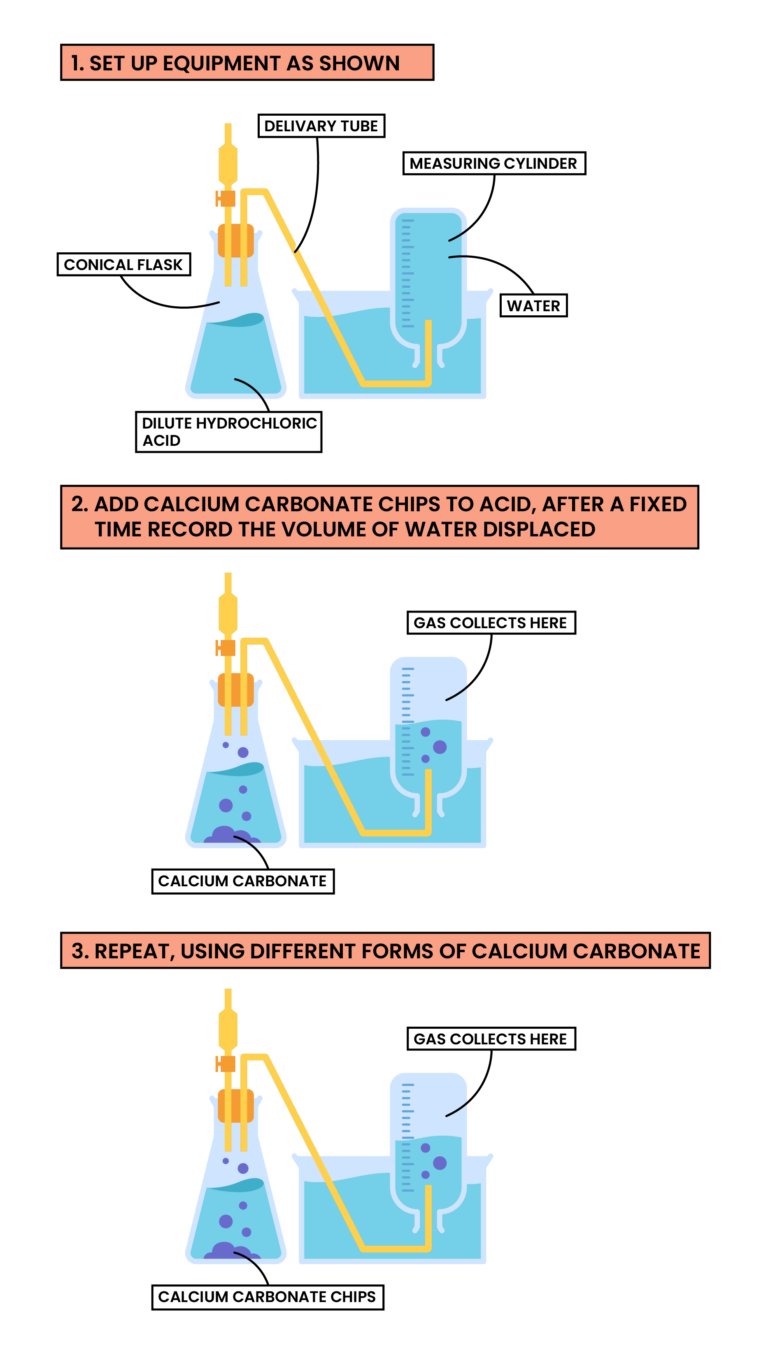
Methods
- Add dilute hydrochloric acid to the conical flask
- Set up the apparatus as shown in the diagram
- Add calcium carbonate chips into the conical flask and close the bung
- Measure the volume of gas produced in a fixed time
- Repeat with different sizes of calcium carbonate chips
Factor 2 Concentration of A Solution
sodium thiosulfate + hydrochloric acid → sodium chloride + sulphur dioxide + sulphur + water
Na2S2O3(aq) + 2HCl(aq) → 2NaCl(aq) + SO2(g) + S(s) + H2O(l)
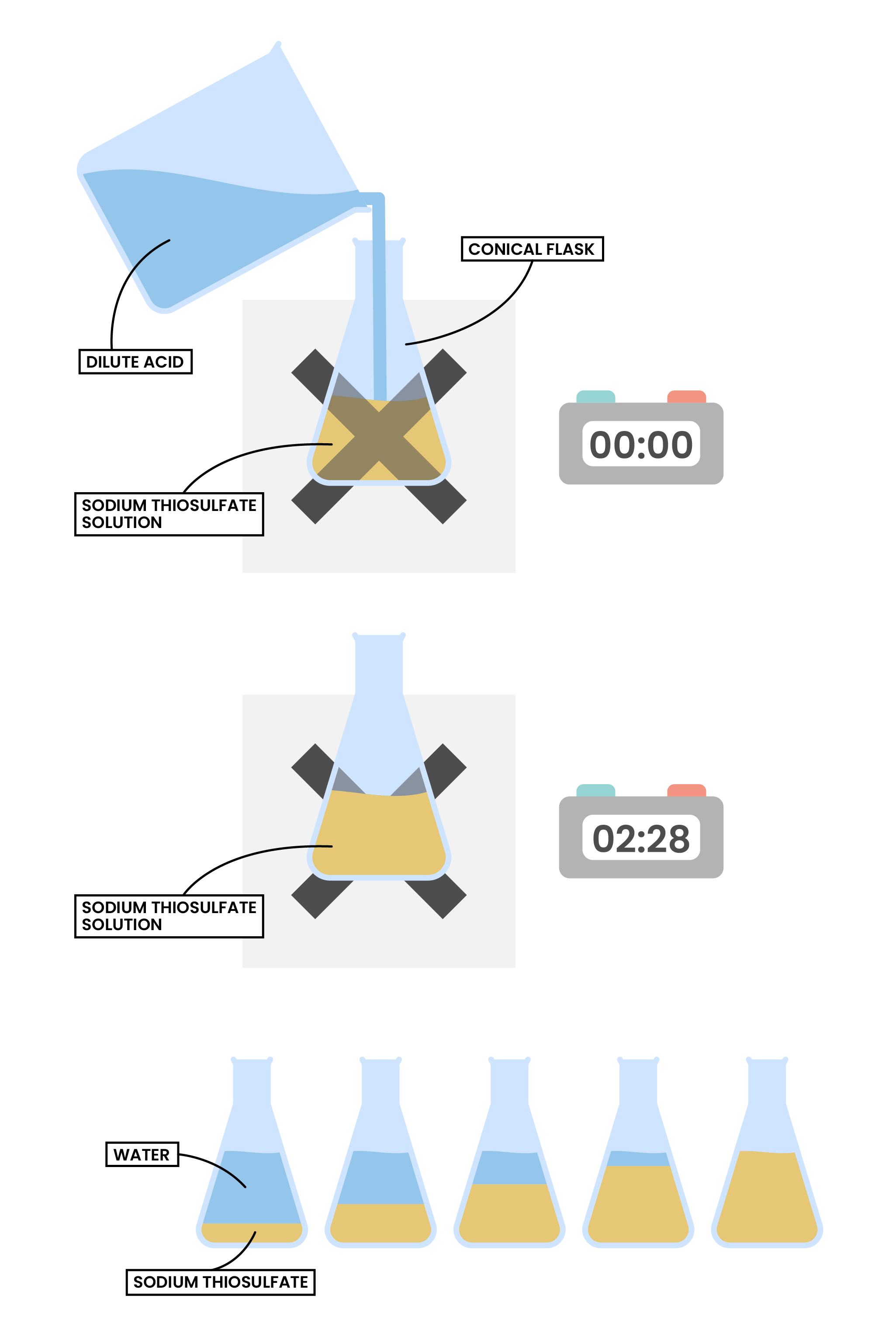
- The reaction changes from transparent to opaque solution
- The reaction flask is placed on a cross drawn on paper
- When the cross disappears, it means a precipitate of sulphur is formed
- Independent variable: concentration of hydrochloric acid solution
- Dependent variable: time for the cross to disappear
- Expected result:
- The higher the concentration of hydrochloric acid solution, the less time needed for the cross to disappear, the higher the rate of reaction
FACTOR 3 TEMPERATURE
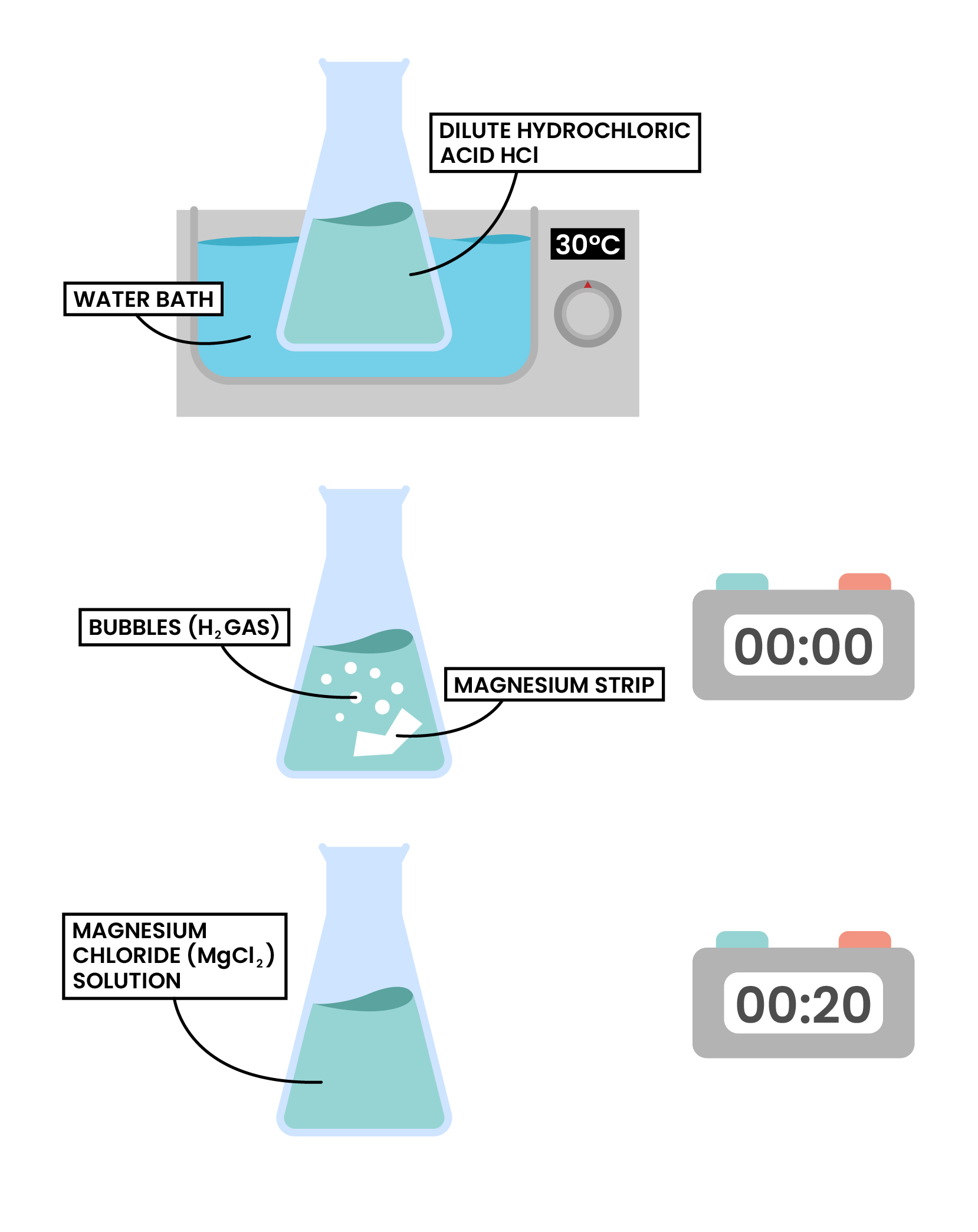
- Any aforementioned reaction could be carried out to investigate the effect of temperature on the rate of reaction
- An example would be a reaction between magnesium and HCl
magnesium + hydrochloric acid → magnesium chloride + hydrogen
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
- Independent variable: temperature of the reaction mixture
- This can be controlled by placing the reaction setup in water bath
- Dependent variable:
- Volume of hydrogen collected in a fixed time OR
-
- Time taken for the magnesium strip to disappear
- Expected result:
-
- The higher the temperature, the less time taken for the magnesium strip to disappear, the higher the rate of reaction
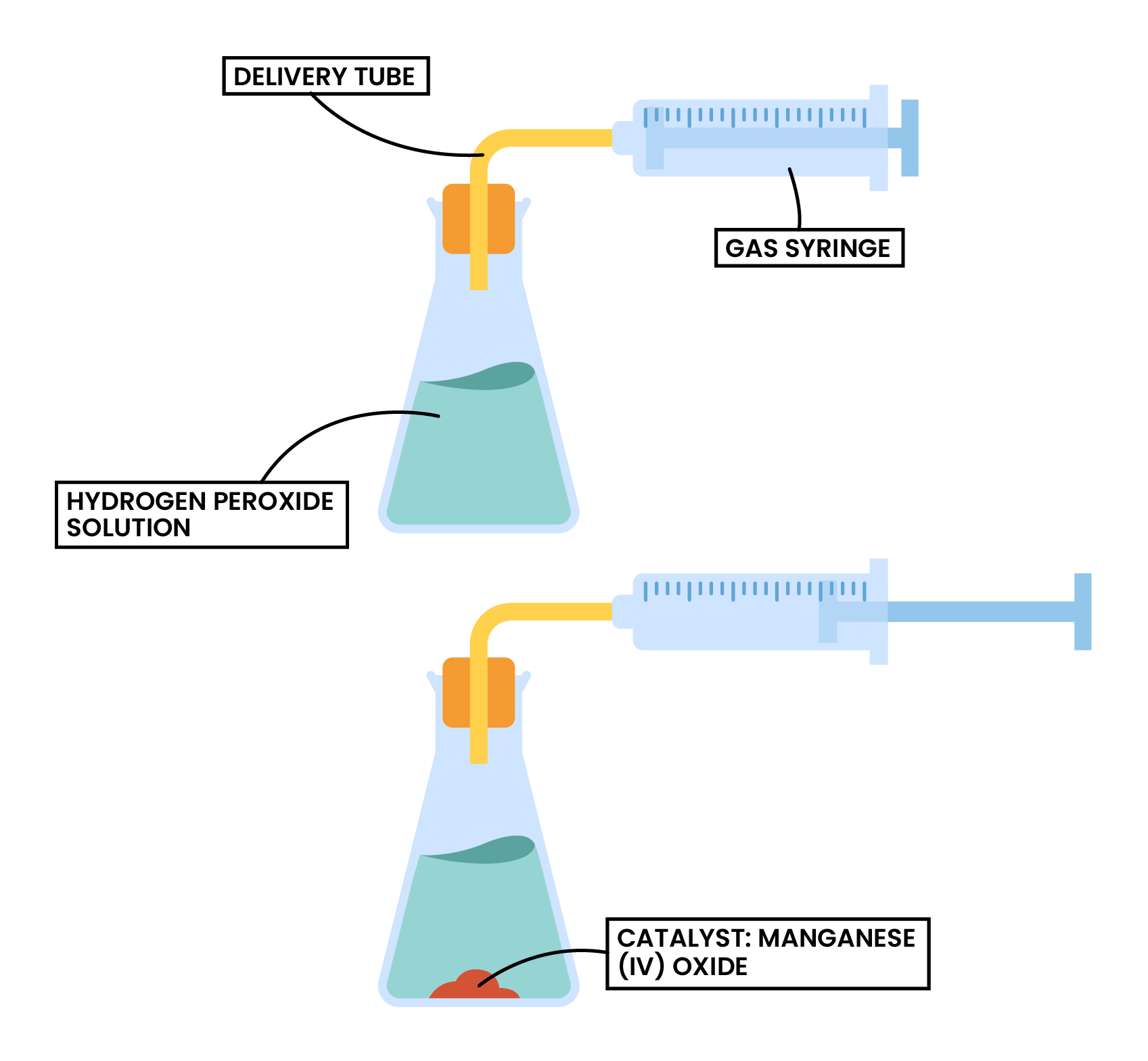
Factor 4 Catalyst
hydrogen peroxide → water + oxygen
2H2O2 (aq) → 2H2O (l) + O2 (g)
- Catalyst needed: manganese (IV) oxide (MnO2)
- Independent variable: with or without catalyst
- Dependent variable: volume of oxygen produced at a fixed time interval, which can be measured by:
- Water displacement method
- Gas syringe method
- Expected result:
- If catalyst MnO2 is added, the volume of oxygen collected after a fixed time interval should be larger, thus higher rate of reaction
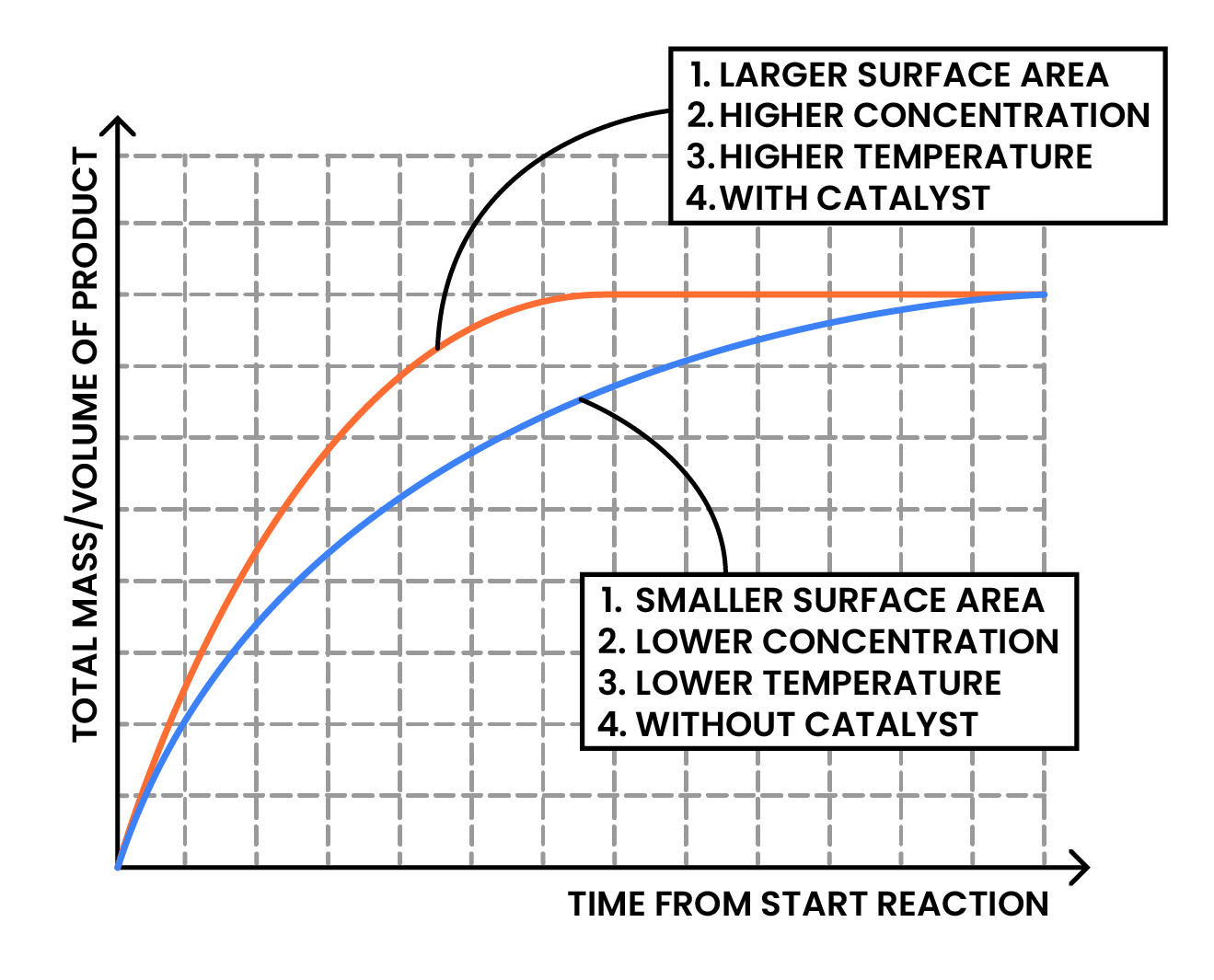
3.2.2 Describe the effects of changes in surface area of a solid, concentration of a solution, pressure of a gas, temperature and the use of a catalyst on the rate of a reaction
Factors affecting rate of reaction:
- Surface area (of solid reactants)
- Concentration (of solutions)
- Pressure (of a gas)
- Pressure of gas depends on the concentration of gas particles, thus the discussion of gas pressure would be combined with concentration of solution
- Temperature
- The use of catalyst
3.2.3 Explain the effects of changes in surface area of a solid, concentration of a solution, pressure of a gas and temperature on the rate of a reaction in terms of particle collision theory
PARTICLE COLLISION THEORY
- Reaction occurs when there are successful collisions among the reactant particles
- Collisions with sufficient energy
- Collisions with correct orientation
- If there is more successful collisions, it means the rate of reaction increases
Effect of Increased Concentration / Increased Pressure
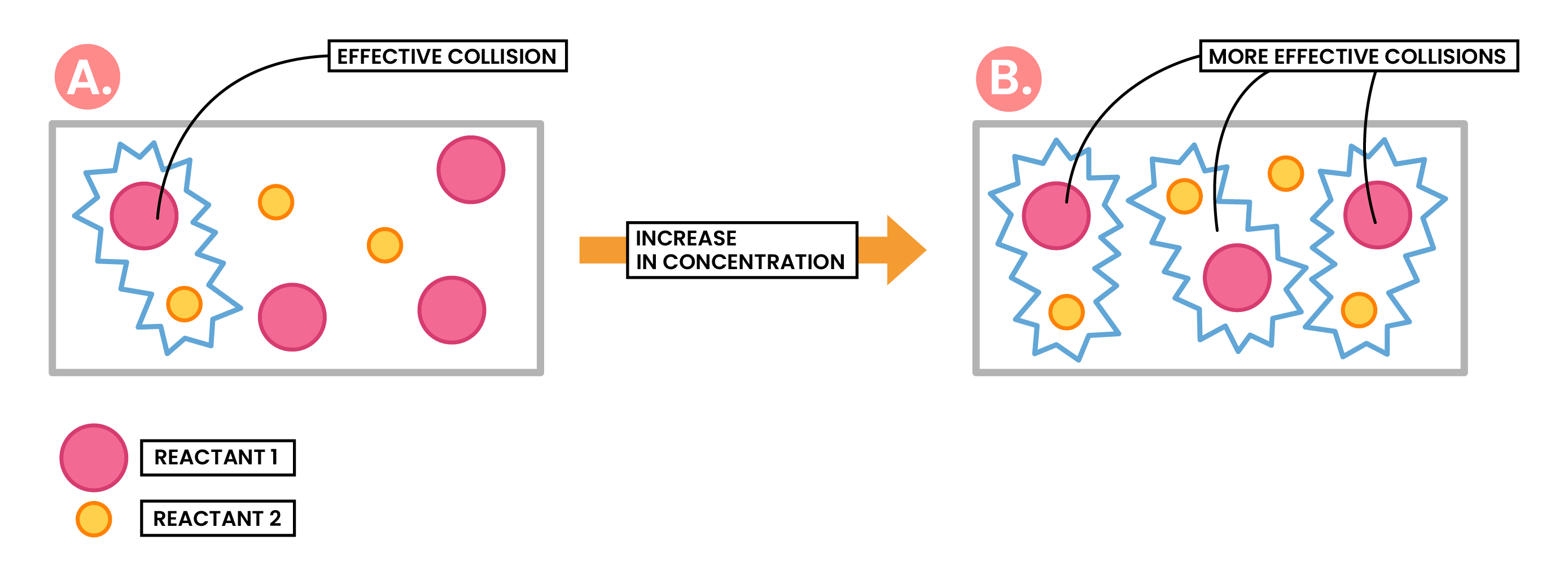
Increasing concentration of a solution
↓
More reactant particles in a given volume
↓
More frequent and successful collisions
↓
Increasing rate of reaction
Effect of Increased Temperature
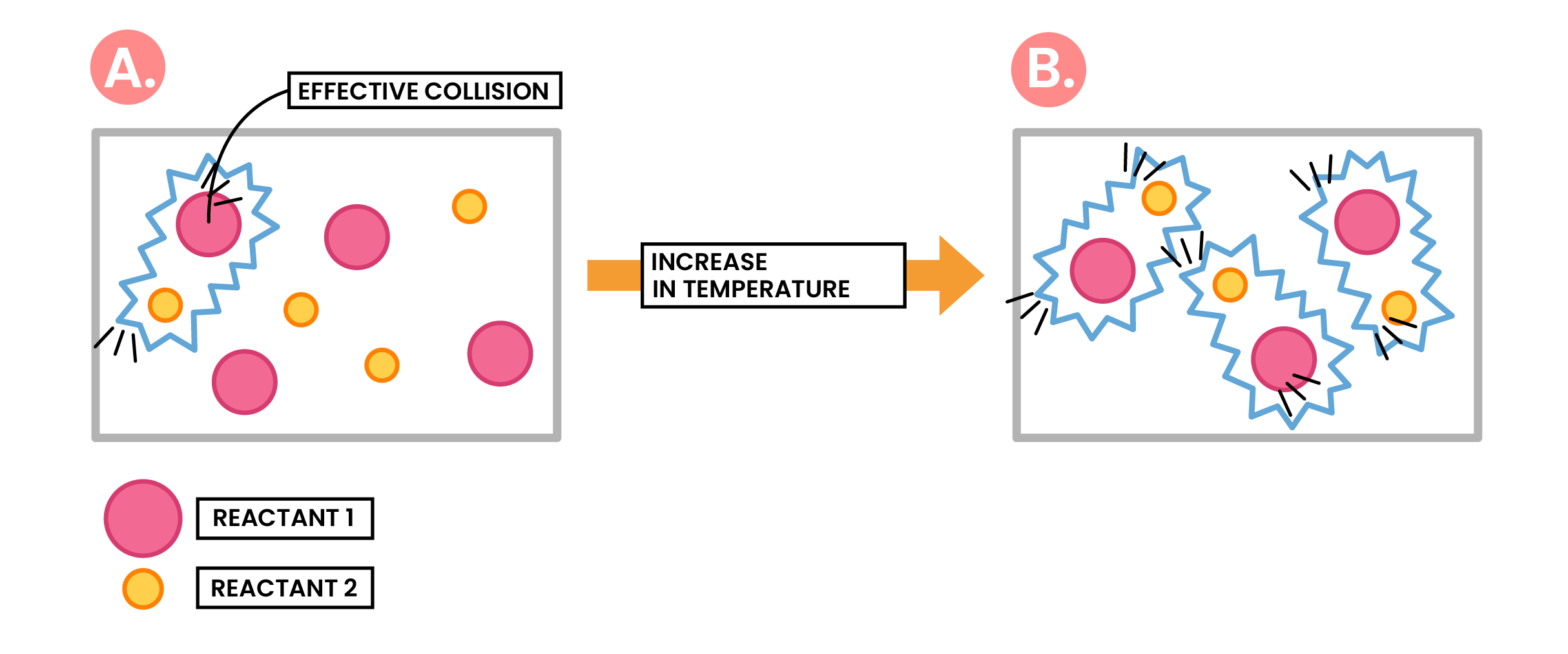
Increasing temperature
↓
Increasing average kinetic energy of particles, higher than required activation energy
↓
More frequent and successful collisions
↓
Increasing rate of reaction
Effect of Increased Surface Area
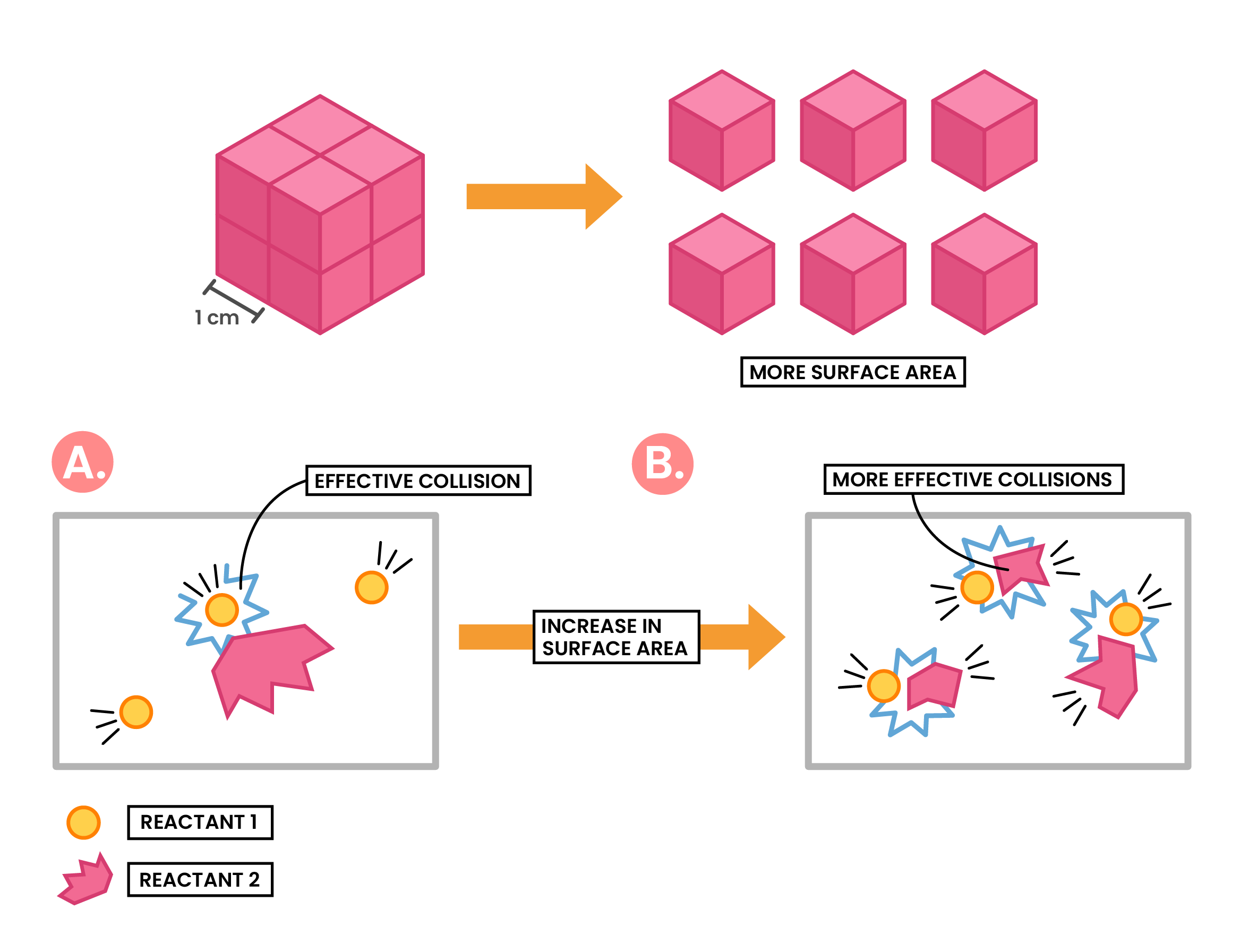
Increasing surface area of solid reactant
↓
More surface area of the particle exposed to other reactant particles
↓
More frequent and successful collisions
↓
Increasing rate of reaction
3.2.4 Know that a catalyst is a substance that increases the rate of a reaction, but is chemically unchanged at the end of the reaction
CATALYST
- Substances that speed up the rate of a reaction
- Catalysts are not altered or consumed in the reaction
- Mass of catalyst is the same at the beginning and end of a reaction
- Thus, they are chemically unchanged at the end of the reaction
3.2.5 Know that a catalyst works by providing an alternative pathway with lower activation energy
ACTIVATION ENERGY (EA)
- To understand how catalysts work, activation energy needs to be discussed
- Activation energy: minimum energy required for a reaction to proceed
CATALYST
- Catalyst provides an alternative pathway with lower activation energy
- By lowering Ea, there are more reactant particles with sufficient energy for effective collisions
- Thus, the rate of reaction increases
3.2.6C Draw and explain reaction profile diagrams showing ΔH and activation energy
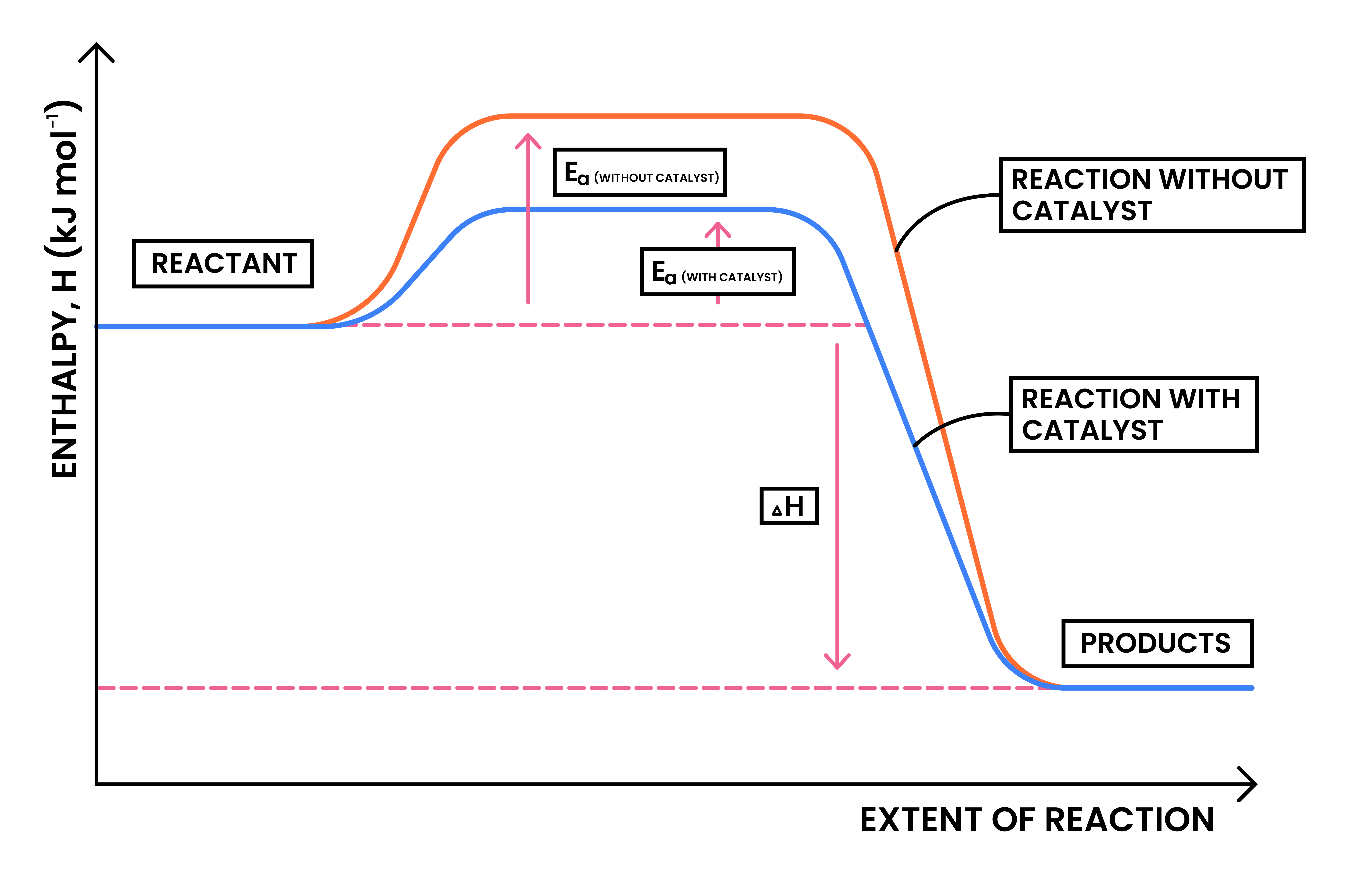
ACTIVATION OF ENERGY (EA)
- Ea is represented in the reaction profile by the difference between reactants and the highest point of the curve
- If catalysts is added, the activation energy is lowered
ΔH
- Represented in the reaction profile by the difference between reactants and products
- ΔH = Energy (products) – Energy (reactants)
- The arrow representing ΔH should be from reactants to products
3.2.7 Practical: investigate the effect of changing the surface area of marble chips and of changing the concentration of hydrochloric acid on the rate of reaction between marble chips and dilute hydrochloric acid
Discussed in 3.1.1
3.2.8 Practical: investigate the effect of different solids on the catalytic decomposition of hydrogen peroxide solution
Discussed in 3.1.1

