REVISION NOTES
IGCSE Edexcel Chemistry
3.3 Reversible Reactions and Equilibria
3.3.1 Know that some reactions are reversible and this is indicated by the symbol ⇌ in equations
COMPLETE REACTION
- Some reactions go to completion
Meaning that: when all reactants are used up to form products, the reaction stops - Indicated by the “→” in the chemical equation
- For example: 2H2O2 → 2H2O + O2
REVERSIBLE REACTION
- Some reactions are reversible
- Meaning that: products can react or decompose and form reactants again
Reaction occurs in both directions- Forward reaction (reactant → product)
- Reverse reaction (product → reactant)
- Indicated by the “⇌” in the chemical equation
- For example: CuSO4・5H2O ⇌ CuSO4 + 5H2O
3.3.2 Describe reversible reactions such as the dehydration of hydrated copper(II) sulfate and the effect of heat on ammonium chloride
Examples of Reversible Reaction
- Dehydration of hydrated copper(II) sulphate
- Thermal decomposition of ammonium chloride
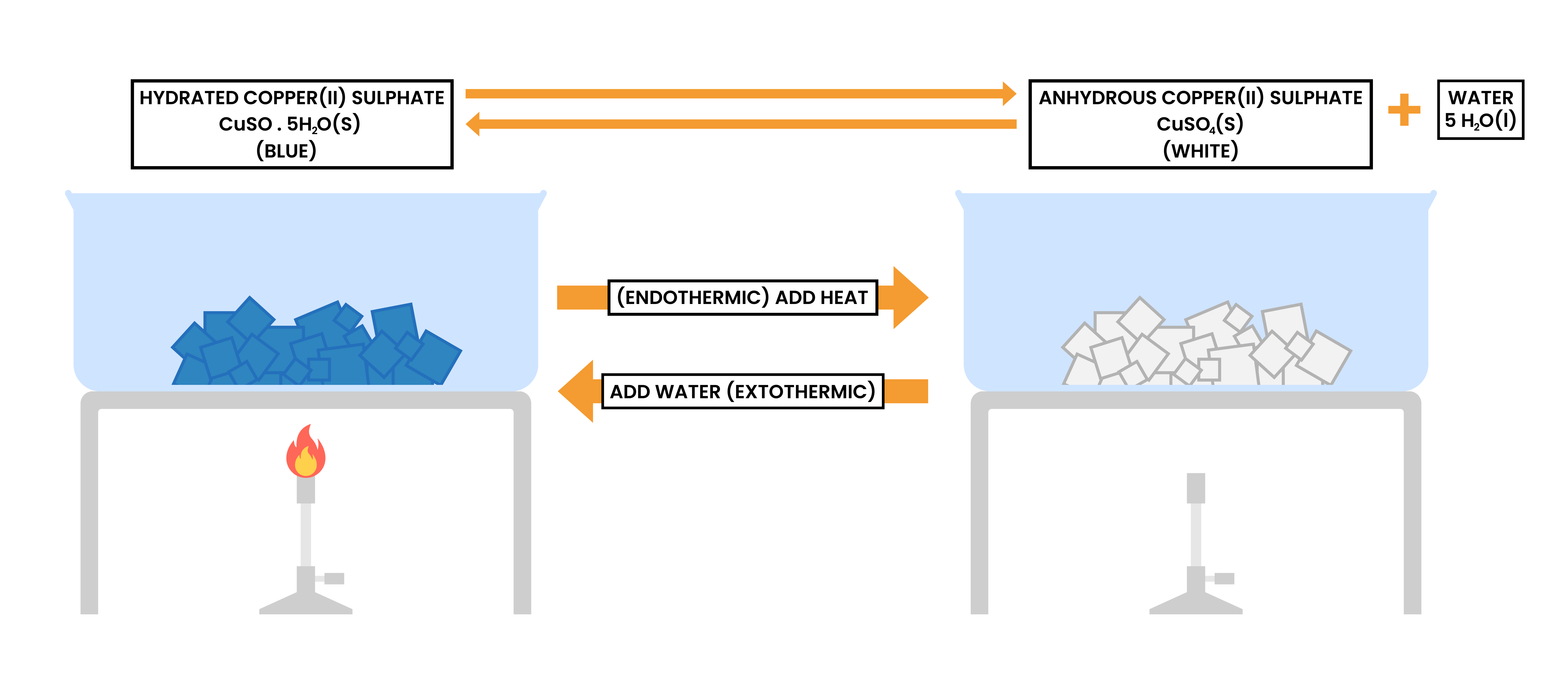
Example 1 Dehydration of Hydrated Copper(II) Sulphate
- When hydrated CuSO4・5H2O crystals are heated in a test tube
- Water is removed
- Blue crystals turn into white powder
- When anhydrous CuSO4 is added to water
- White powder turns blue
- Heat is given off (reaction is exothermic)
CuSO4・5H2O (s) ⇌ CuSO4 (s) + 5H2O (l)
EXAMPLE 2 THERMAL DECOMPOSITION OF AMMONIUM CHLORIDE
- Heating ammonium chloride produces ammonia and hydrogen chloride gases
NH4Cl (s) → NH3 (g) + HCl (g)
- As the hot gases cool down they recombine to form solid ammonium chloride
NH3 (g) + HCl (g) → NH4Cl (s)
ammonium chloride ⇌ ammonia + hydrogen chloride
NH4Cl (s) ⇌ NH3 (g) + HCl (g)
3.3.3C Know that a reversible reaction can reach dynamic equilibrium in a sealed container
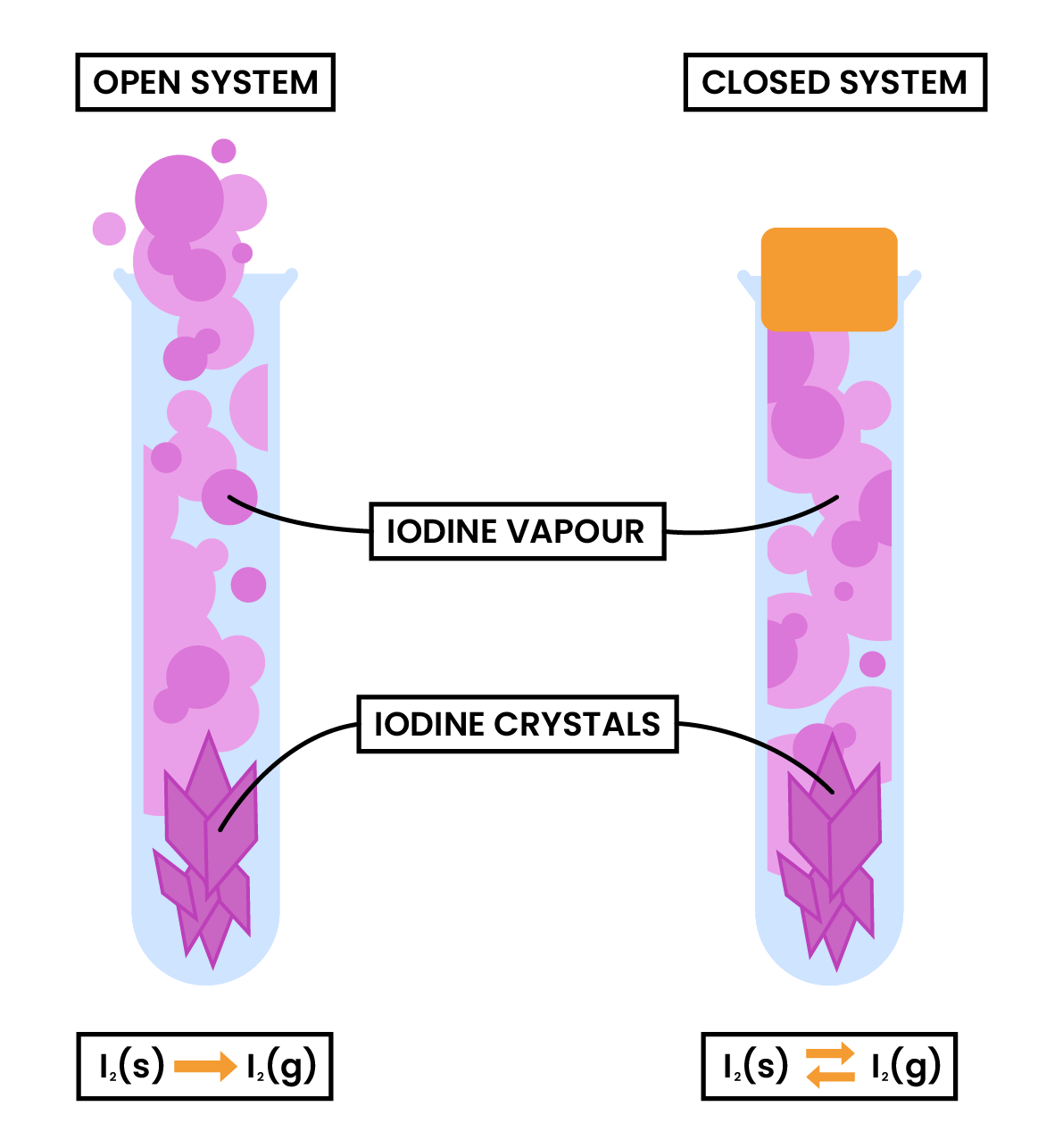
DYNAMIC EQUILIBRIUM
- Dynamic equilibrium can be reached when a reversible reaction is taken place in a closed system
- None of the participating chemical substances are able to leave the container
3.3.4C Know that the characteristics of a reaction at dynamic equilibrium are:
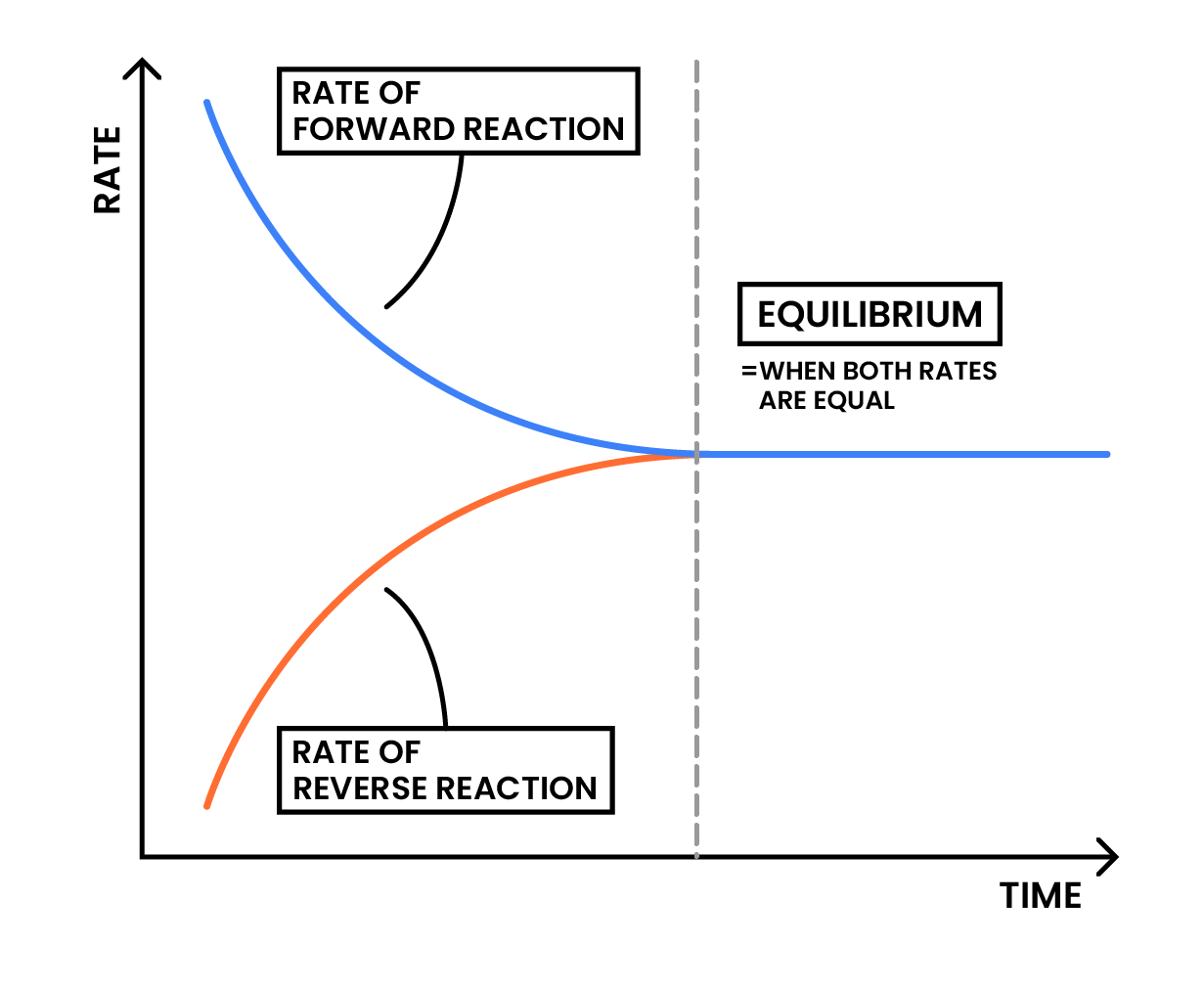
- The forward and reverse reactions occur at the same rate
- The concentrations of reactants and products remain constant.
When a reaction is at dynamic equilibrium:
- Both forward and reverse reactions are occuring at the same rate
- Therefore, the net change of the system is constant, and the concentrations of reactants and products respectively remain constant
- Dynamic equilibrium does not mean the concentrations of reactants and products are the same
- Dynamic equilibrium does not mean the reaction stops, both forward and reverse reactions are still happening.
3.3.5C Understand why a catalyst does not affect the position of equilibrium in a reversible reaction
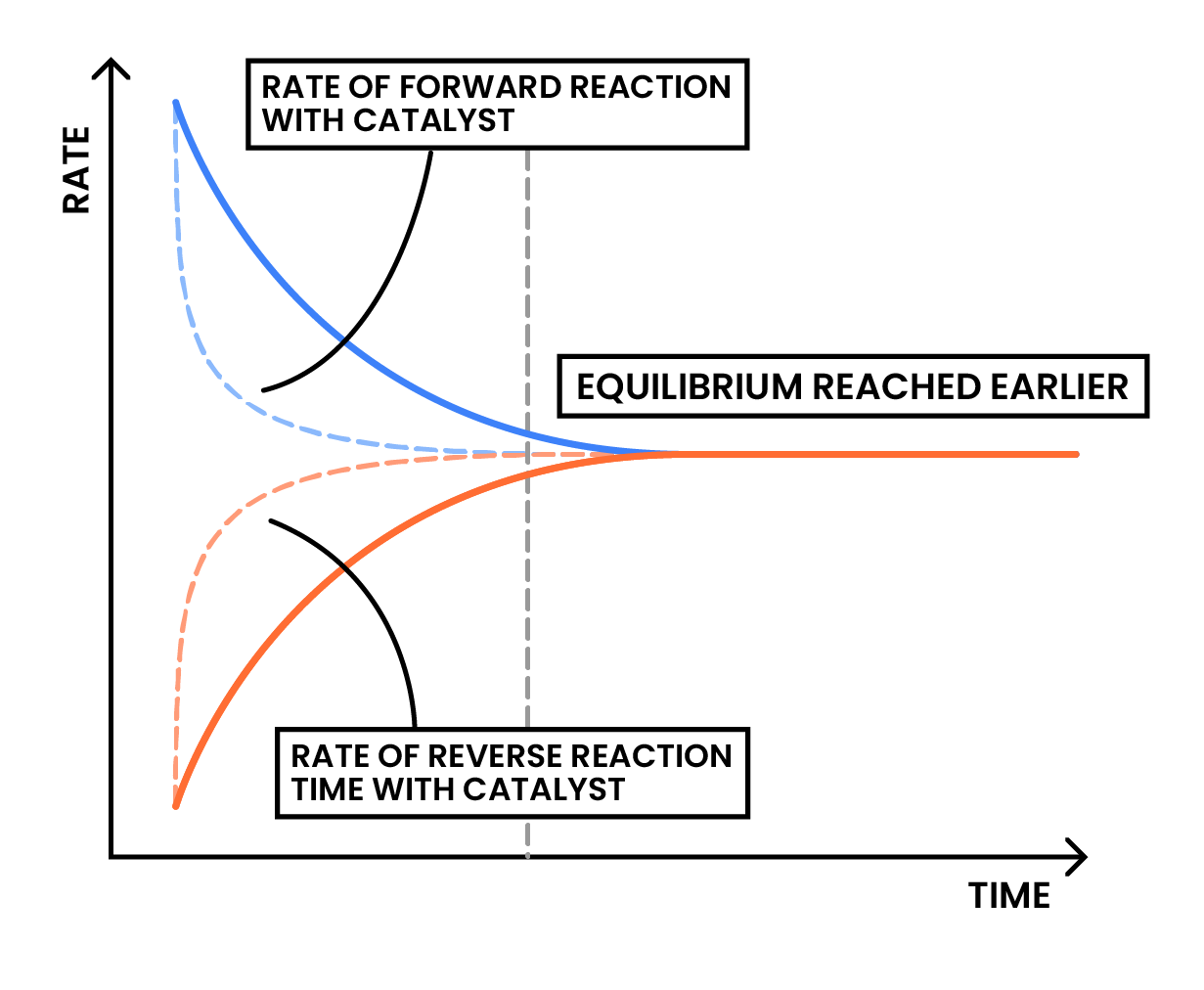
Presence of catalyst does not affect the position of equilibrium
- Catalyst increases the rate of reaction
- In this case, it increases the rate of both forward and reverse reactions
- It does not change the concentration of reactants and products at equilibrium
- Position of equilibrium is not affected
3.3.6C Know the effect of changing either temperature or pressure on the position of equilibrium in a reversible reaction:
- An increase (or decrease) in temperature shifts the position of equilibrium in the direction of the endothermic (or exothermic) reaction
- An increase (or decrease) in pressure shifts the position of equilibrium in the direction that produces fewer (or more) moles of gas
References to Le Chatelier’s principle are not required
- The system would respond to oppose the change
- This is known as Le Chatelier’s principle (not required)
- Factors to be discussed:
- Temperature
- Pressure
Effect of Temperature
Example
ICl + Cl2 ⇌ ICl3
Dark brown Yellow
When the equilibrium mixture is heated, it becomes dark brown in colour.
HOW DO WE KNOW WHETHER THE BACKWARD REACTION IS EXOTHERMIC OR ENDOTHERMIC?
- Equilibrium has shifted to the left as the colour dark brown means that more of ICI is produced
- Increasing temperature moves the equilibrium in the endothermic direction
- So the backward reaction is endothermic
Effect of Pressure
Example
2NO2 ⇌ N2O4
Brown gas Colourless gas
WHAT IS THE EFFECT OF AN INCREASE IN PRESSURE ON THE POSITION OF EQUILIBRIUM?
- Number of gas molecules on the left = 2
- Number of gas molecules on the right = 1
- An increase in pressure will cause equilibrium to shift in the direction that produces the smaller number of molecules of gas (2 → 1)
- So equilibrium shifts to the right

