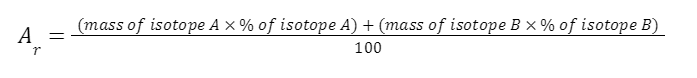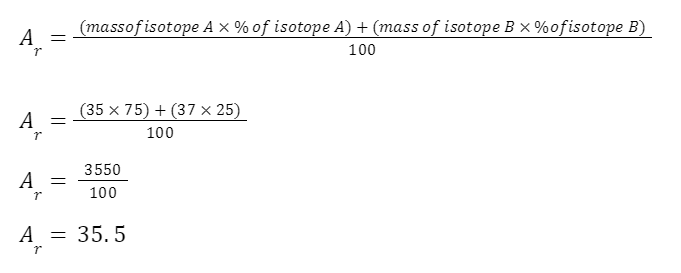REVISION NOTES
IGCSE Edexcel Chemistry
1.3 Atomic Structure
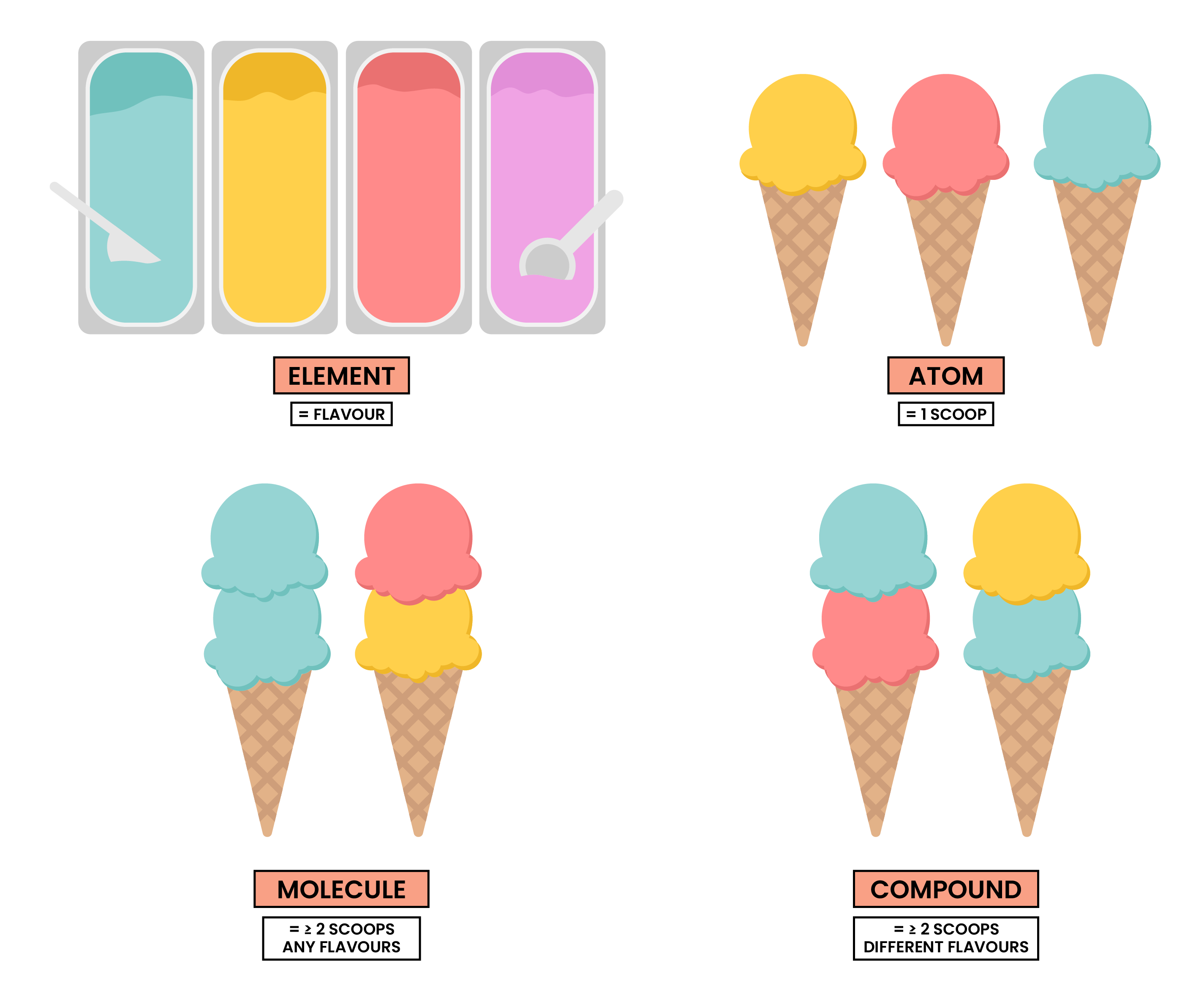
1.3.1 Know what is meant by the terms atom and molecule

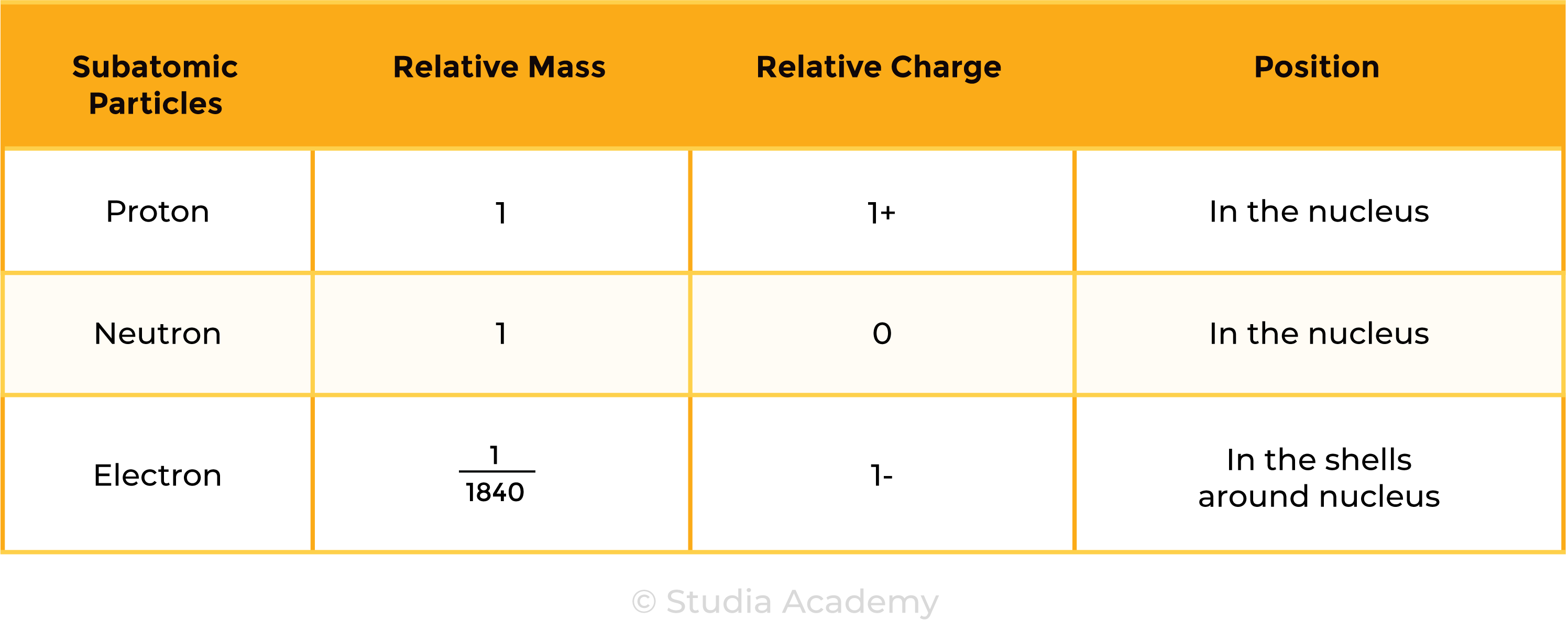
1.3.2 Know the structure of an atom in terms of the positions, relative masses and relative charges of subatomic particles
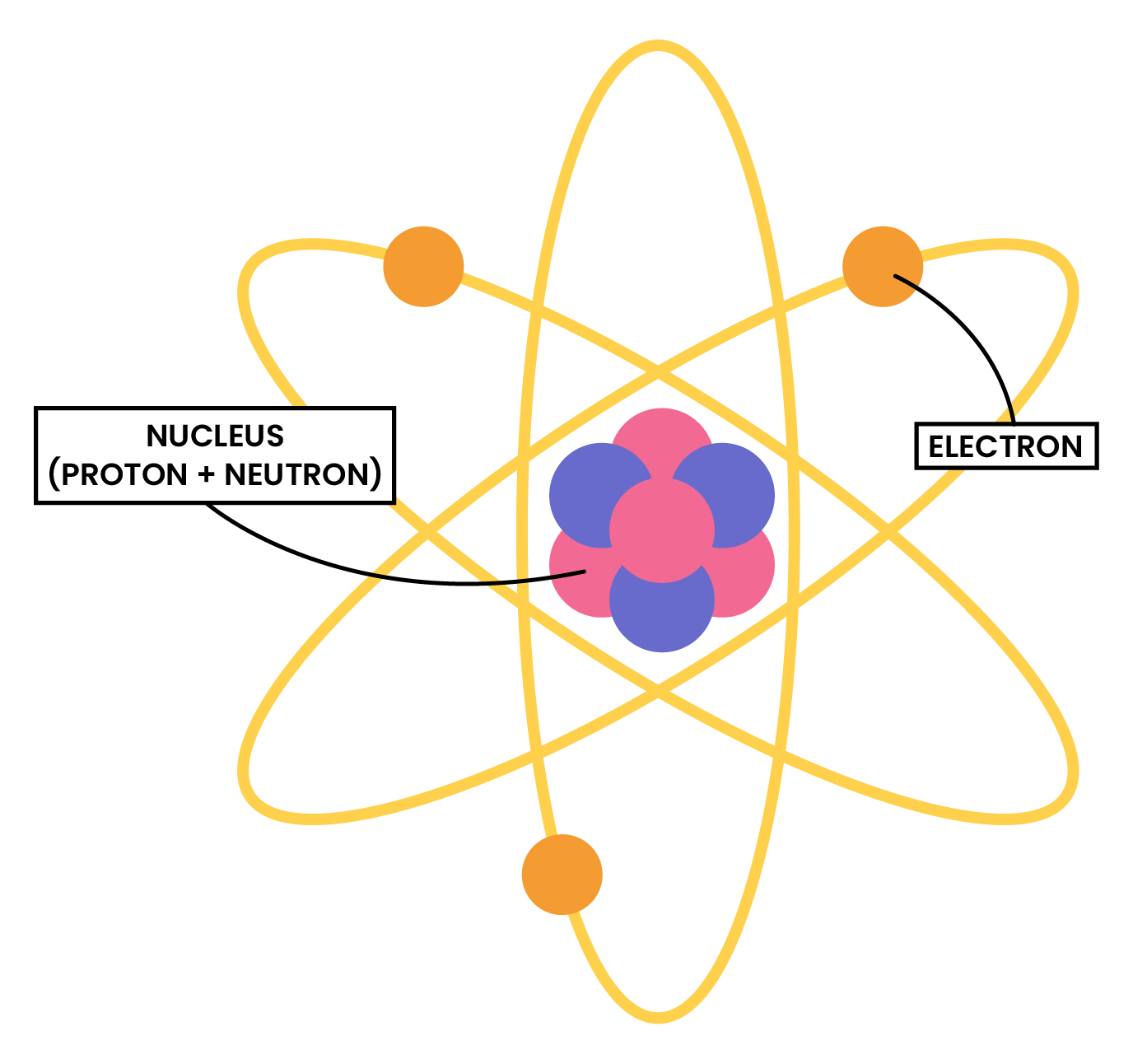
1.3.3 Know what is meant by the terms atomic number, mass number, isotopes and relative atomic mass (Ar)
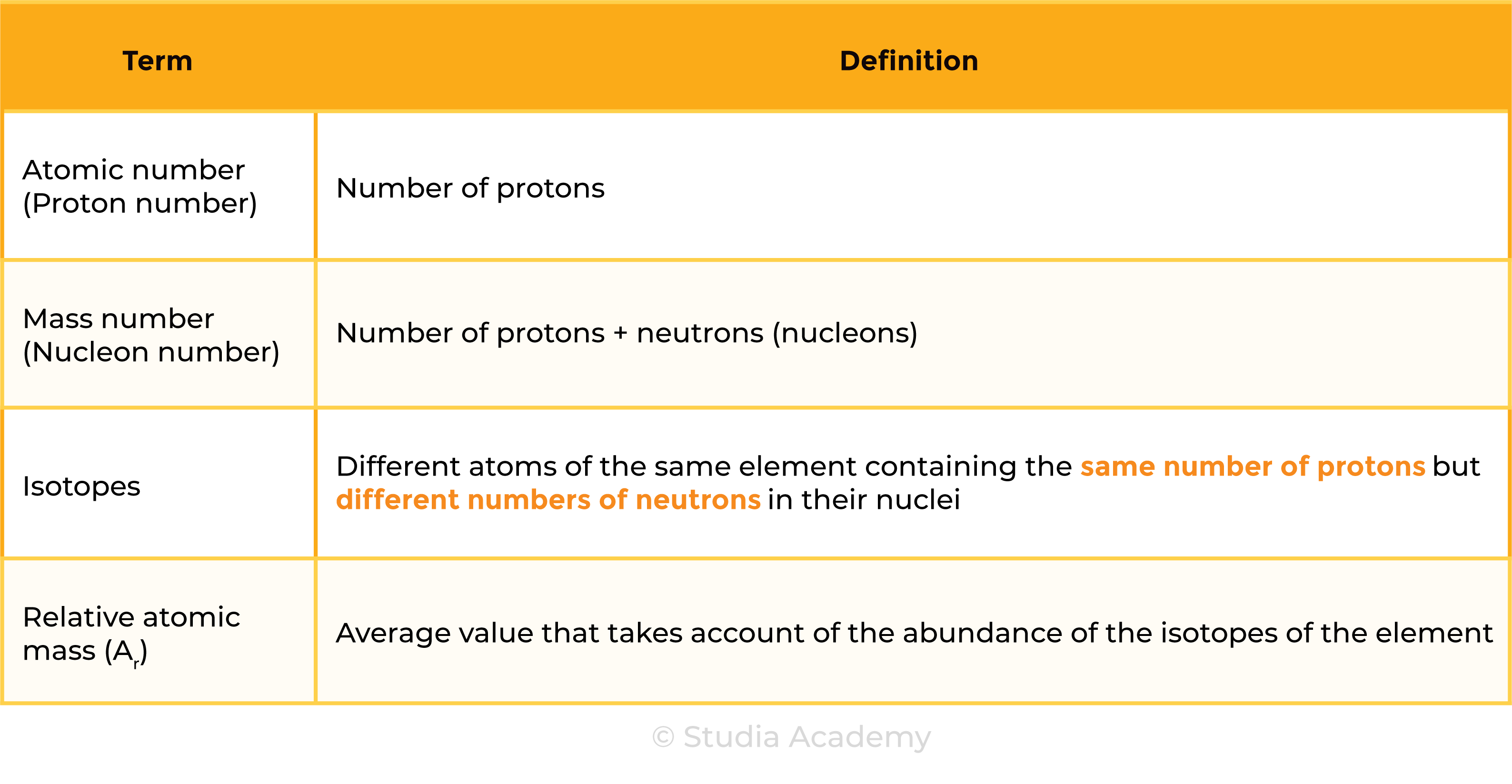
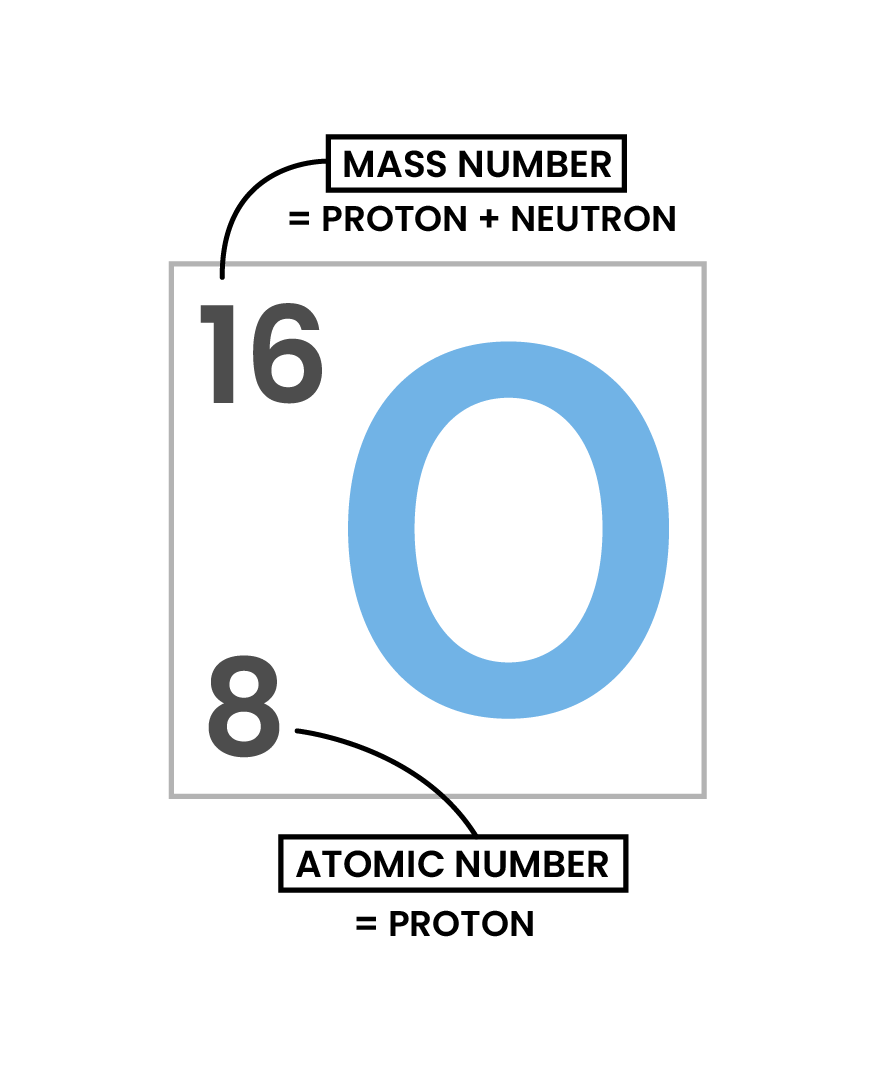
- Number of protons = atomic number
- Number of electrons =
- For atoms: number of protons = number of electrons (Because atom has overall zero charge)
-
- For ions: number of protons ≠ number of electrons
- Number of neutrons = mass number – atomic number
ISOTOPES
- Atoms of same element; same number of protons; different number of neutrons
- They have identical chemical properties, as they have same number of electrons
- Slightly different physical properties
1.3.4 Be able to calculate the relative atomic mass of an element (Ar) from isotopic abundances
ISOTOPIC ABUNDANCE
- Isotopic abundance: percentage of the isotope found in a naturally occurring sample
The relative atomic mass of each element is calculated from the mass number and relative abundances of all the isotopes of a particular element
EXAMPLE
A sample of chlorine gas is a mixture of 2 isotopes, chlorine-35 and chlorine-37. These isotopes occur in specific proportions in the sample i.e. 75% chlorine-35 and 25% chlorine-37. Calculate the relative atomic mass of chlorine in the sample.