REVISION NOTES
IGCSE Edexcel Chemistry
1.6 Ionic Bonding
1.6.1 Understand how ions are formed by electron loss or gain
IONS
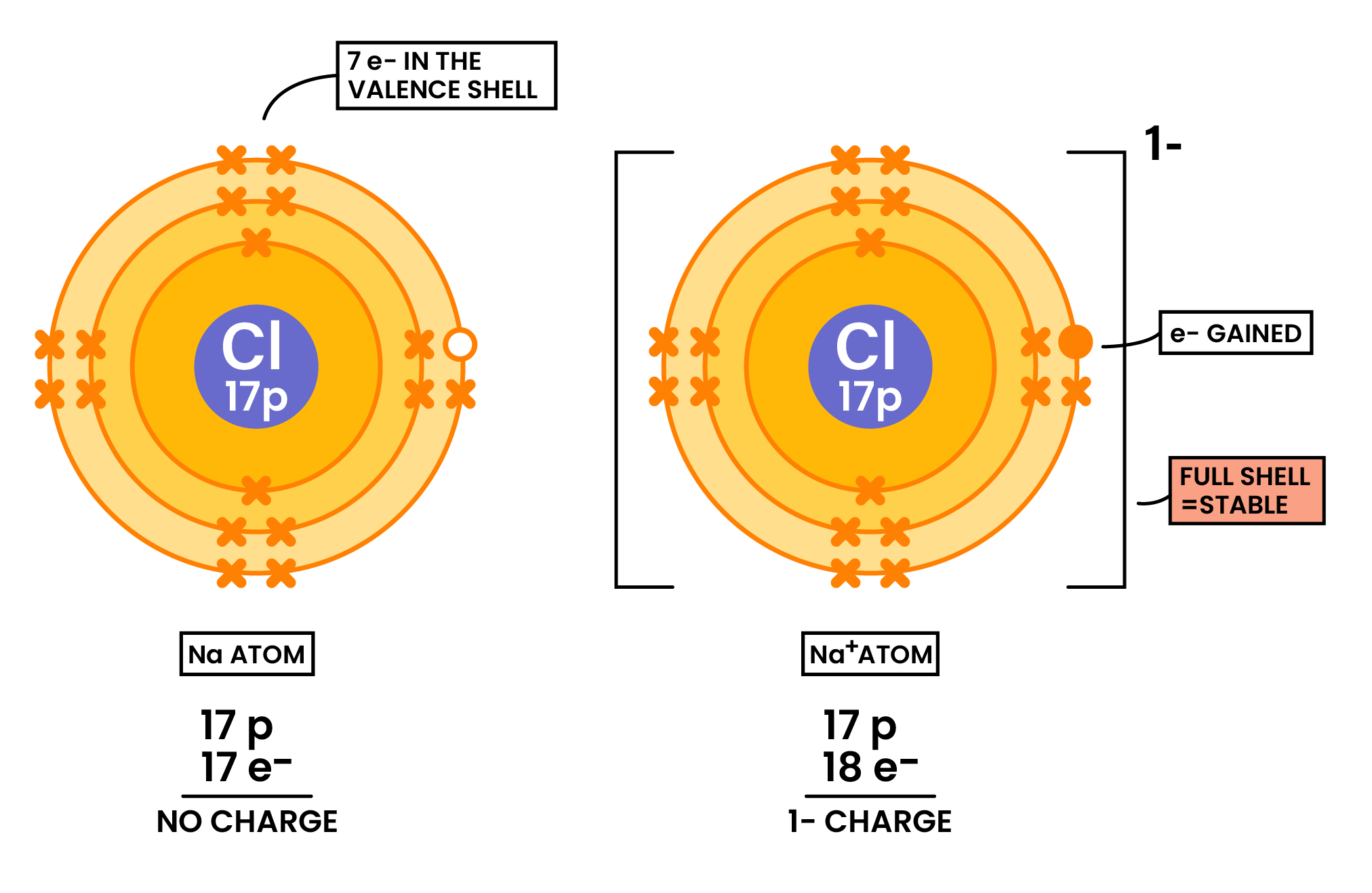
- Ions are formed when atoms gain or lose electron(s)
- In order to form full electron shells (octet rule)
- So that it can be more stable
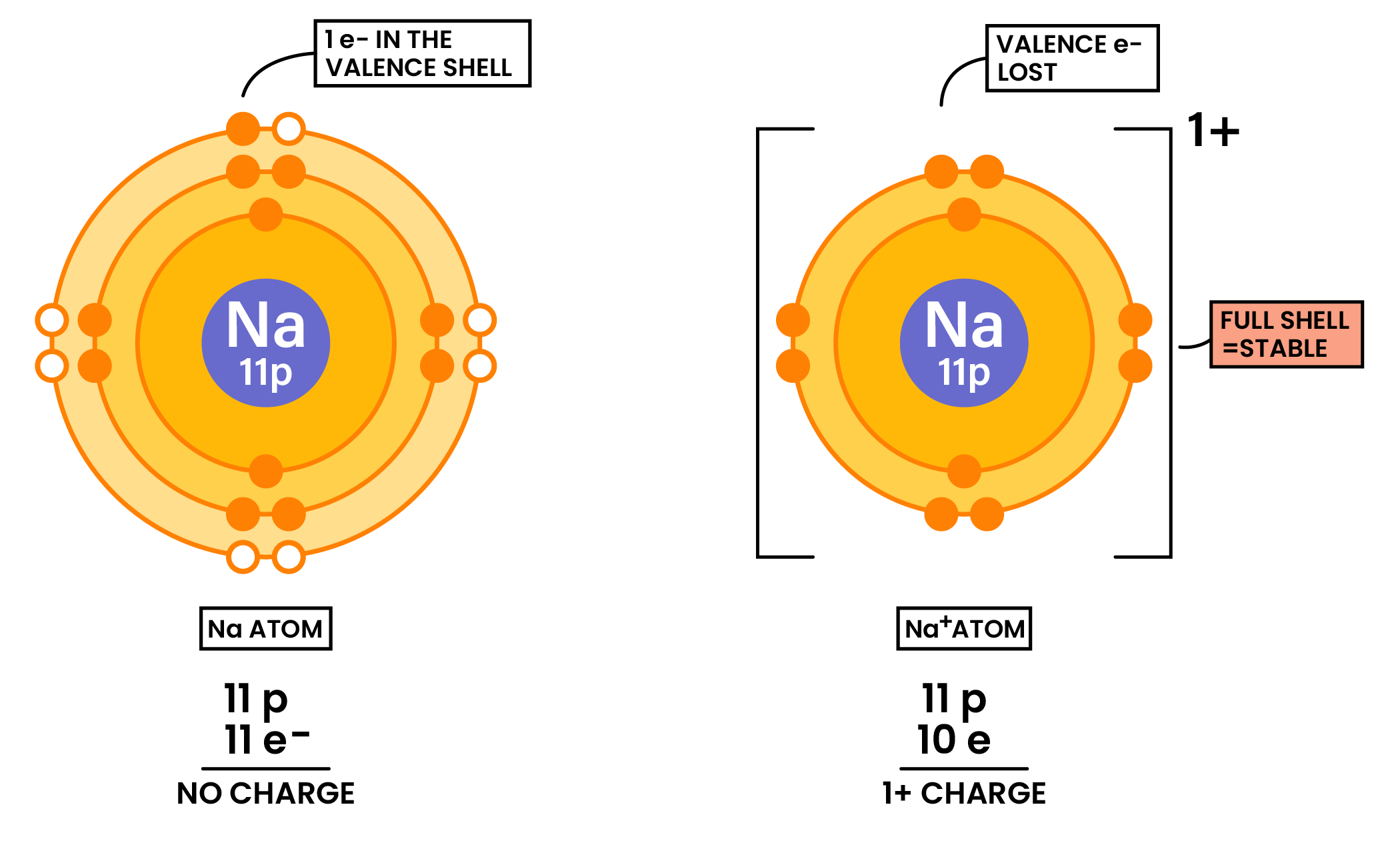
- Metals tend to lose electrons → forming positive ions (cations)
- Nonmetals tend to gain electrons → forming negative ions (anions)
Formation of Cations (Metal Losing Electrons)
Formation of Anions(Nonmetal Gaining Electrons)
1.6.2 Know the charges of these ions:
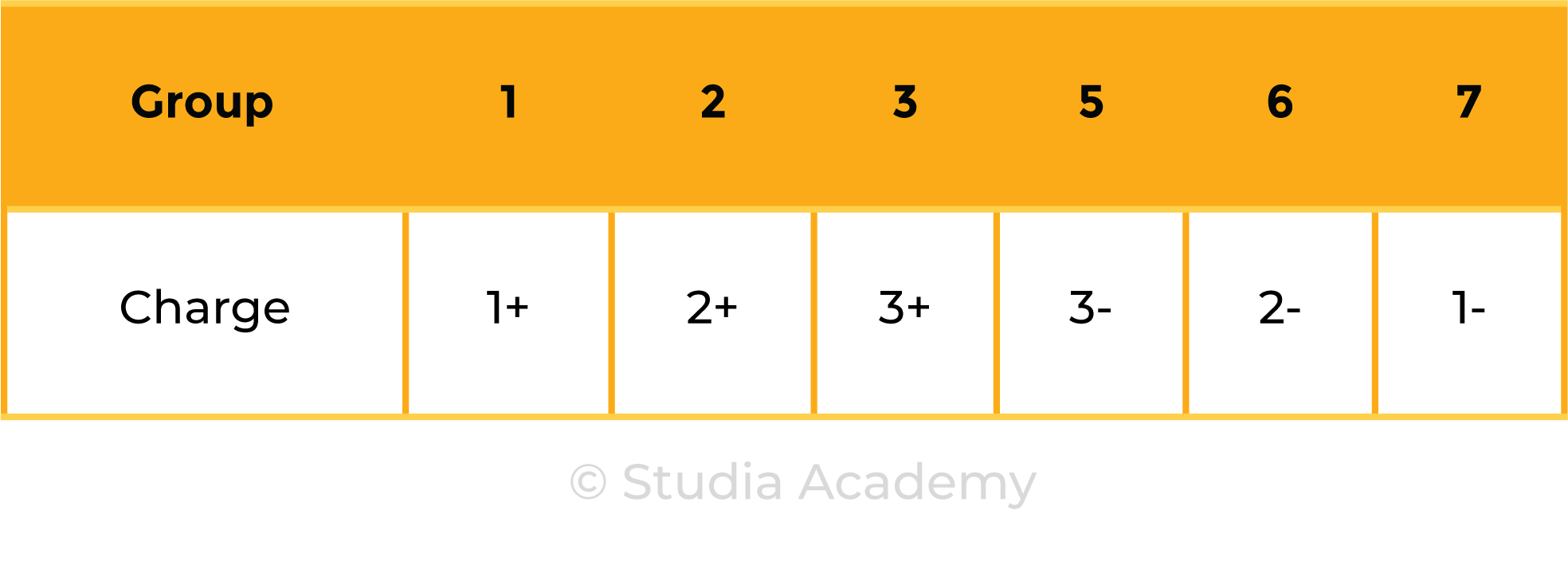
- Metals in Groups 1, 2 and 3
- Non-metals in Groups 5, 6 and 7
- Ag+, Cu2+, Fe2+, Fe3+, Pb2+, Zn2+
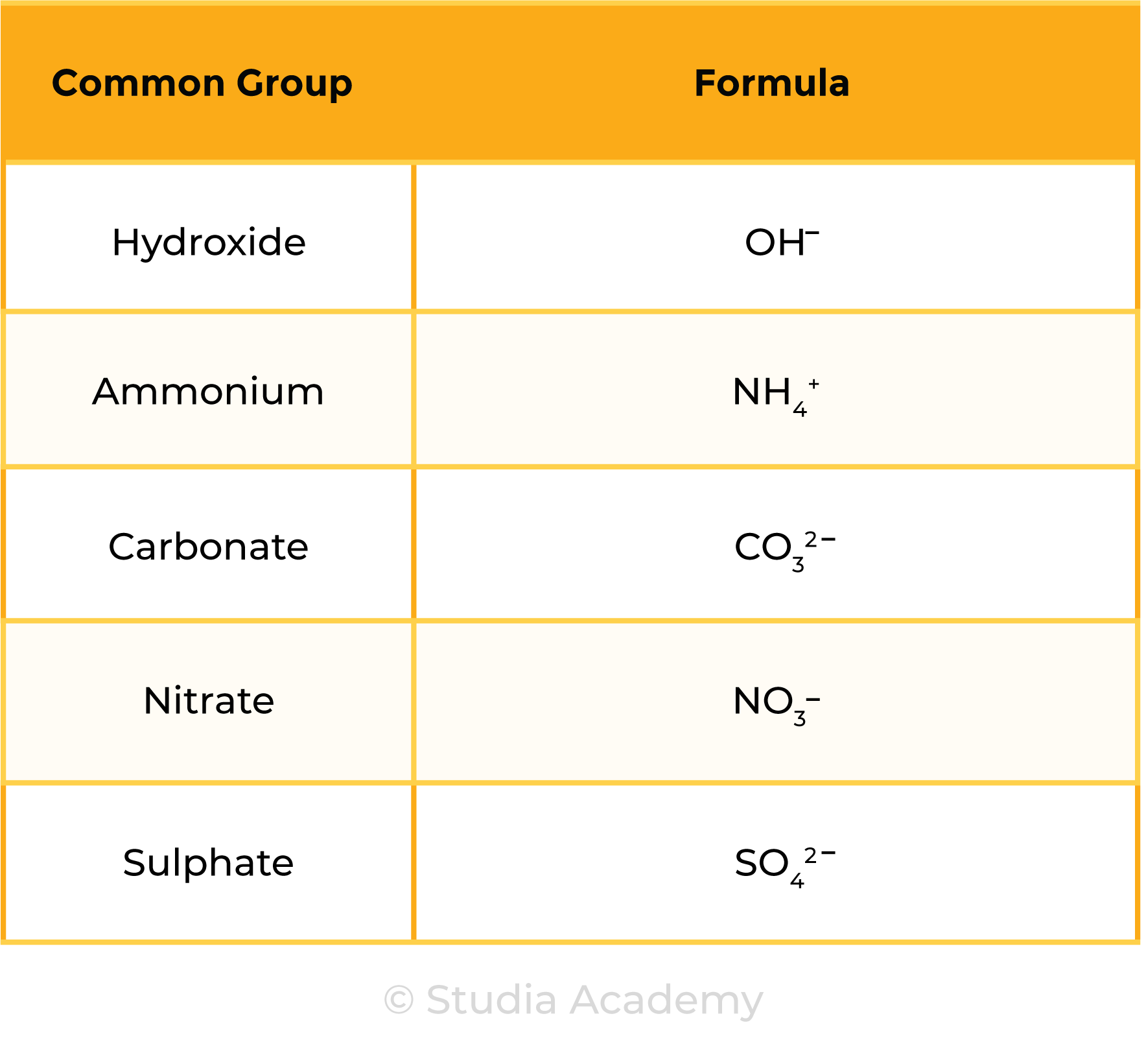
- Hydrogen (H+), Hydroxide (OH–), Ammonium (NH4+), Carbonate (CO32–), Nitrate (NO3–), Sulphate (SO42–).
Metals and Nonmetals in Group 1, 2, 3, 5, 6 and 7:
Common Groups (Polyatomic Ions):
1.6.3 Write formulae for compounds formed between the ions listed above
IONIC COMPOUNDS
- Ionic compounds are formed when a positive ion combines with a negative ion
- The net charge of an ionic compound is 0
- Number of ions should be multiplied to make sure the sum of charge = 0
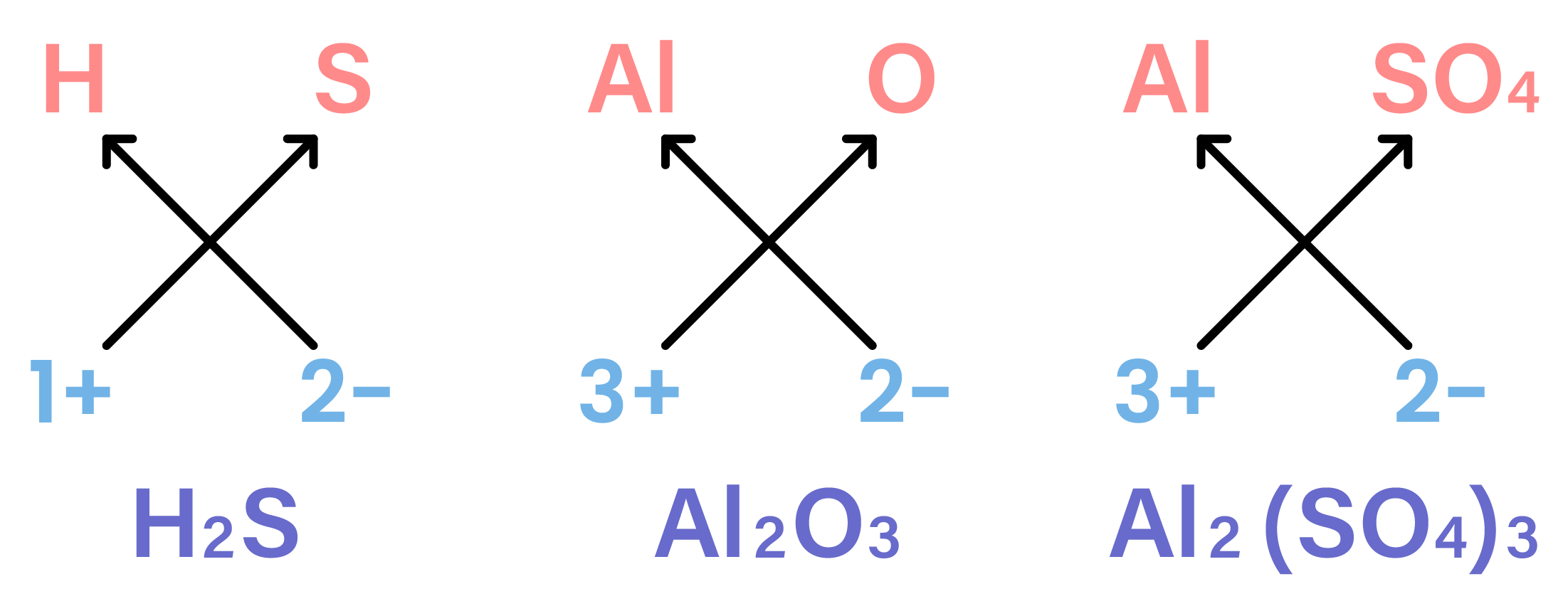
- Cross-multiply each ion with the charge of the other ion
- It can be applied to common groups (polyatomic ions) as well
EXAMPLE
What is the formula of aluminium sulphate?
Al is in group III
→ charge = 3+
→ Al3+
Sulphate is a common ion
→ SO42-
1.6.4 Draw dot-and-cross diagrams to show the formation of ionic compounds by electron transfer, limited to combinations of elements from Groups 1, 2, 3 and 5, 6, 7 only outer electrons need be shown
Ionic compounds are formed when there is electron transfer between metals and nonmetals
- Metals lose electrons
- Nonmetals gain electrons
- Electron(s) gained by the nonmetal should be annotated by a different symbol than the symbols of electrons in the nonmetal
- After ions are formed, there should be square bracket and charge on the top right corner
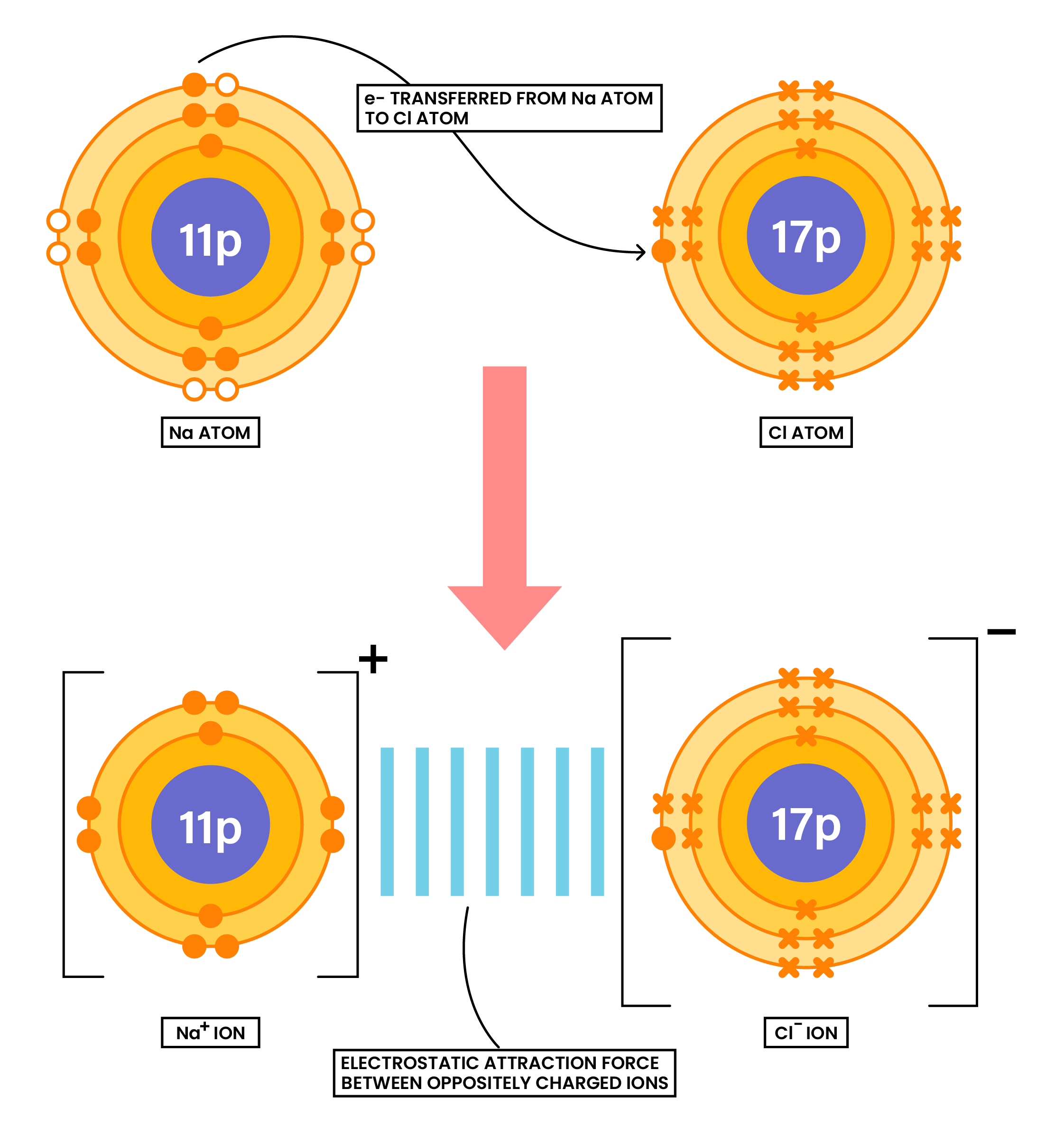
EXAMPLE
Draw the dot-and-cross diagram to show the formation of the compound CaO
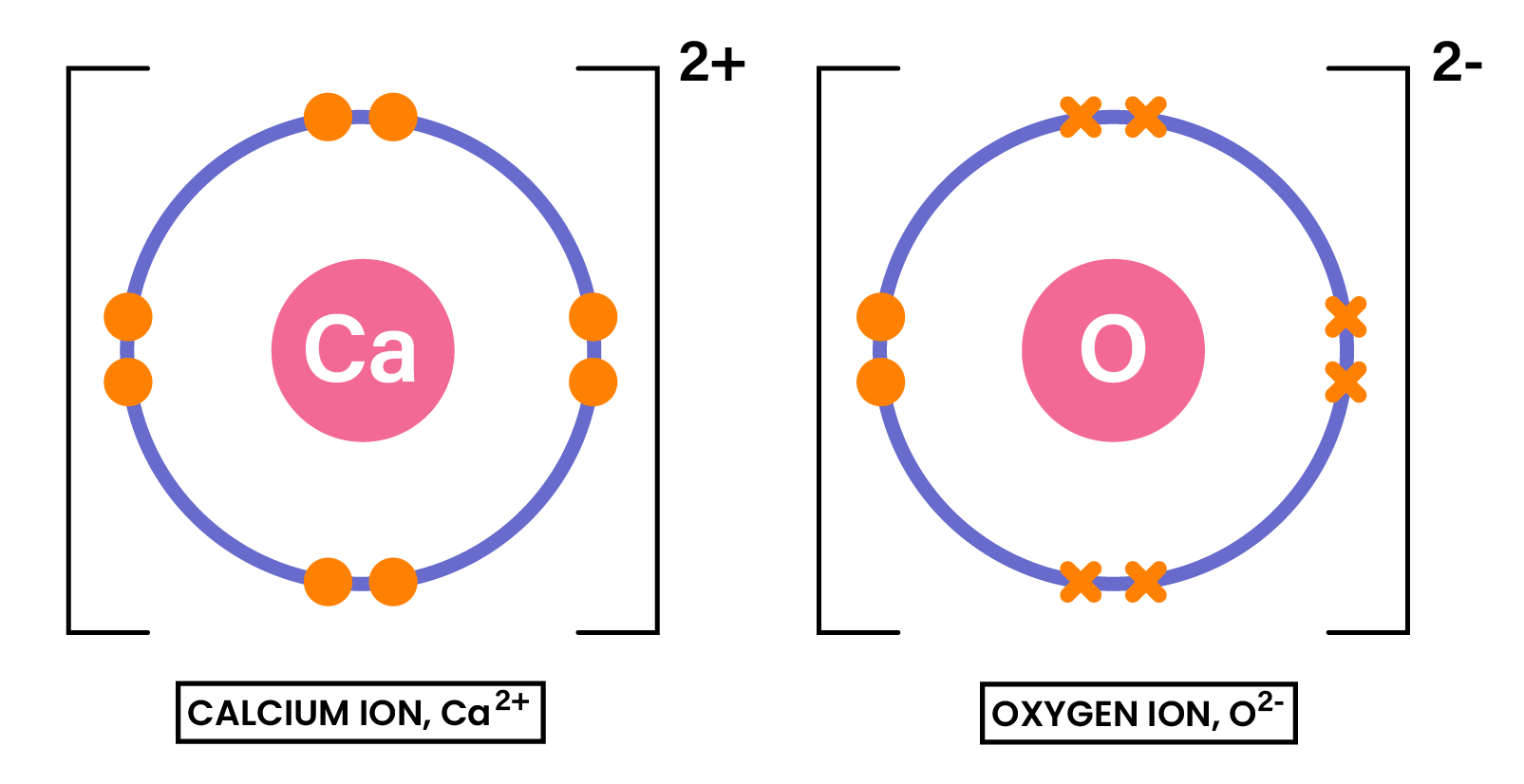
1.6.5 Understand ionic bonding in terms of electrostatic attractions
IONIC BONDING
- Electrostatic attraction force between two oppositely charged ions
- Exact terminologies should be memorised
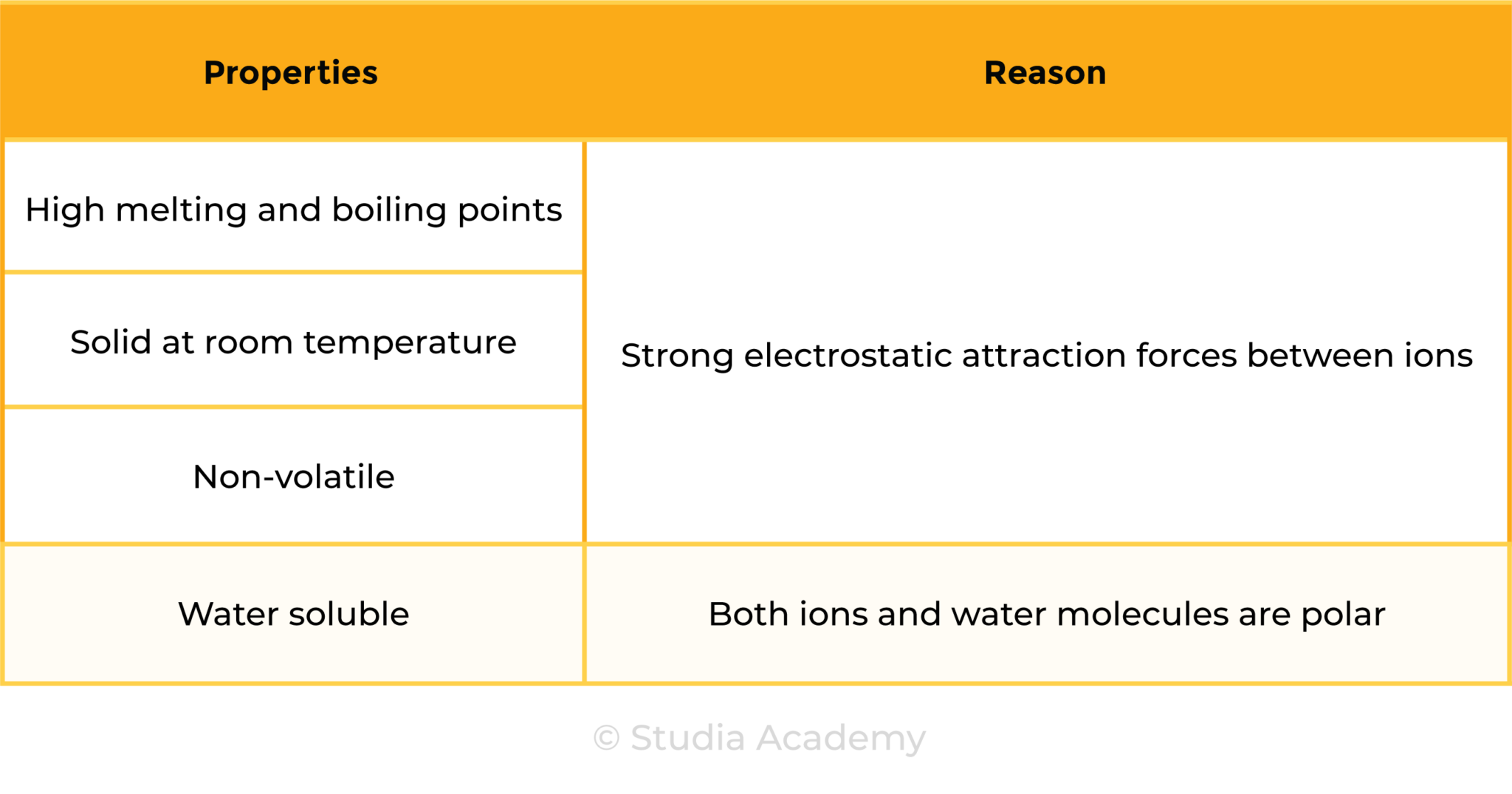
1.6.6 Understand why compounds with giant ionic lattices have high melting and boiling points
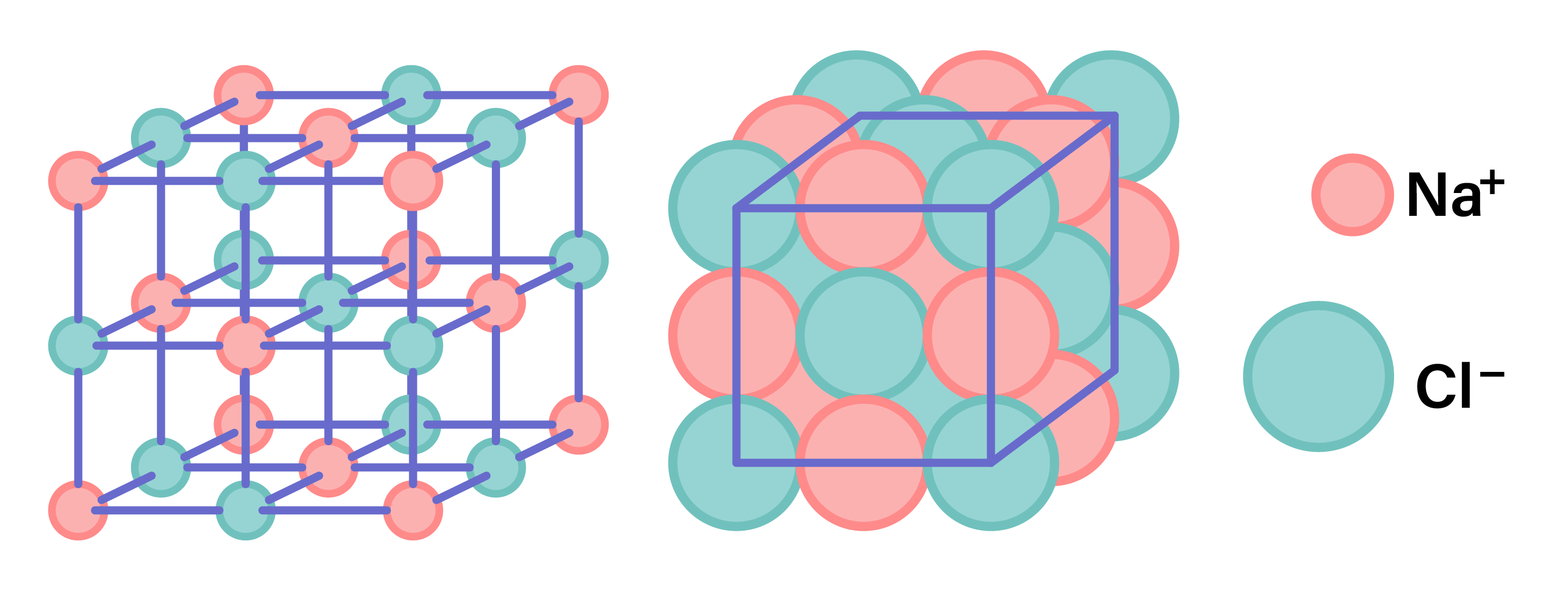
STRUCTURE OF IONIC COMPOUNDS
- Ionic compounds are giant lattice structure of ions
- Due to the strong electrostatic attraction forces, ionic compounds have high melting and boiling points
- Ionic compounds are usually solid at room temperature and are non-volatile
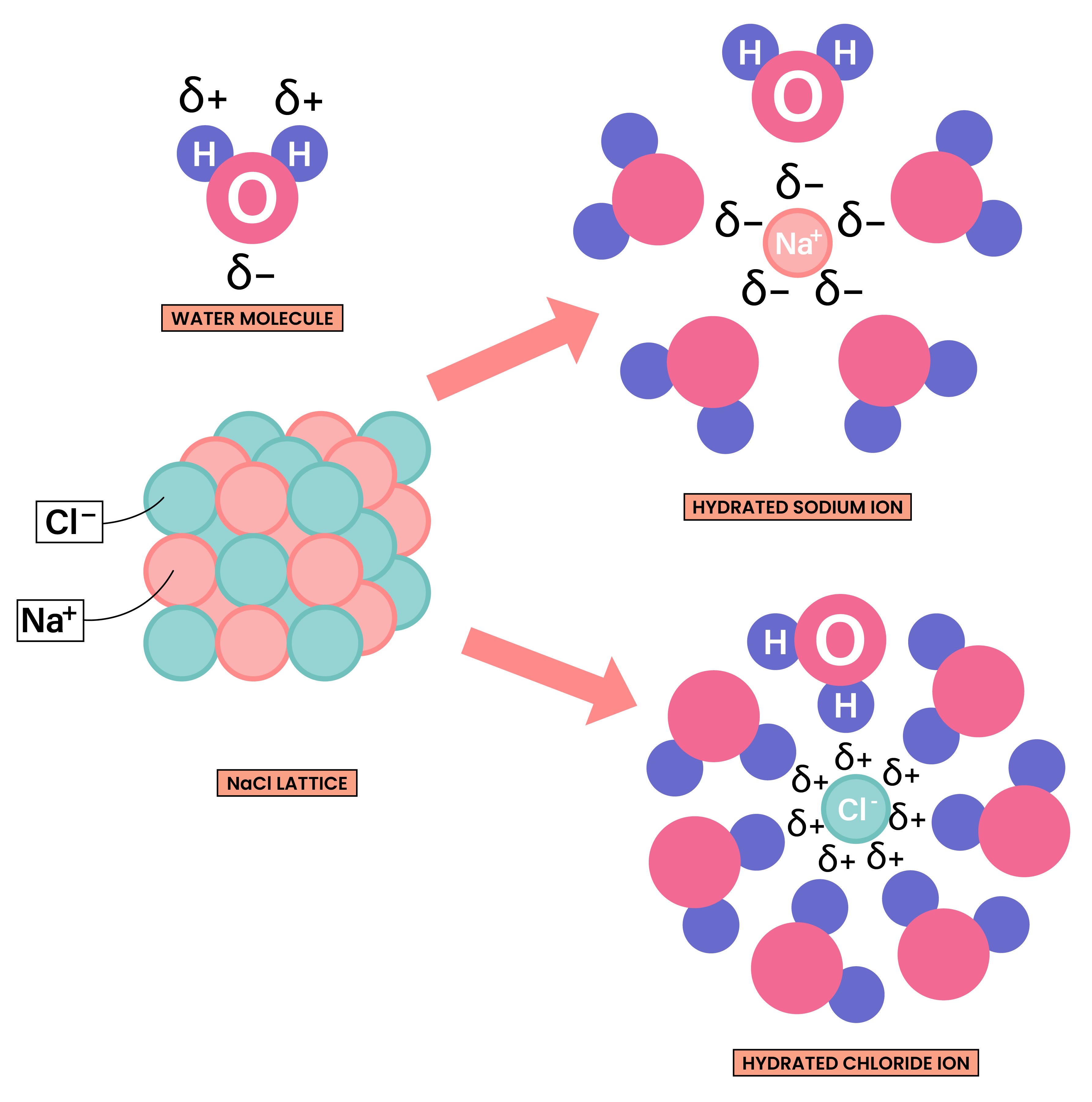
- They are usually water soluble as both ionic compounds and water are polar substances
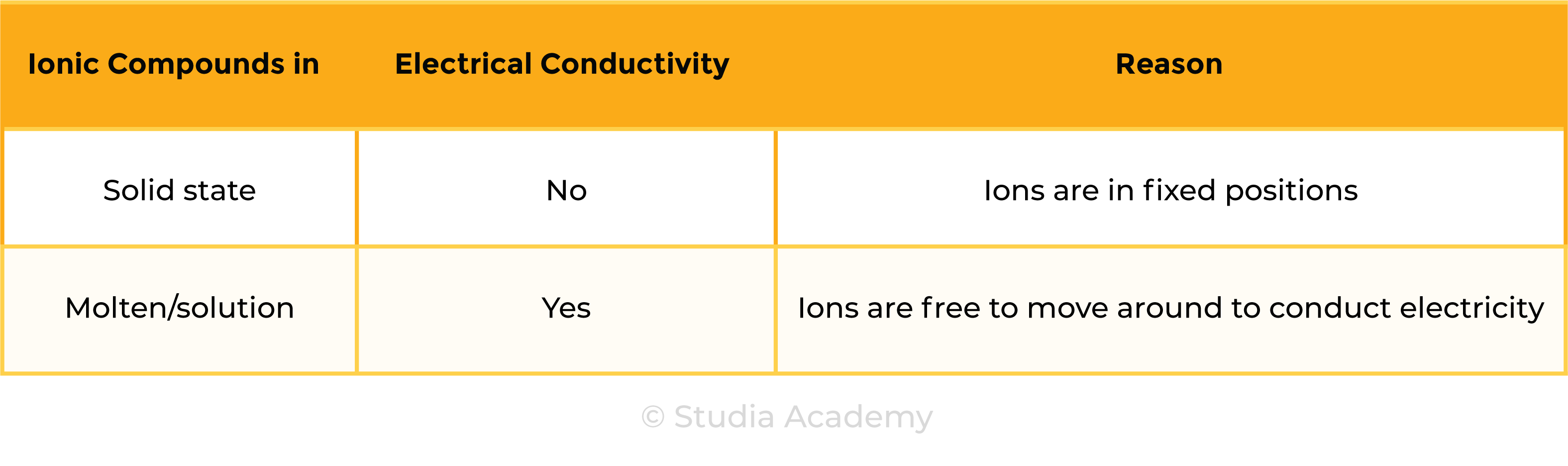
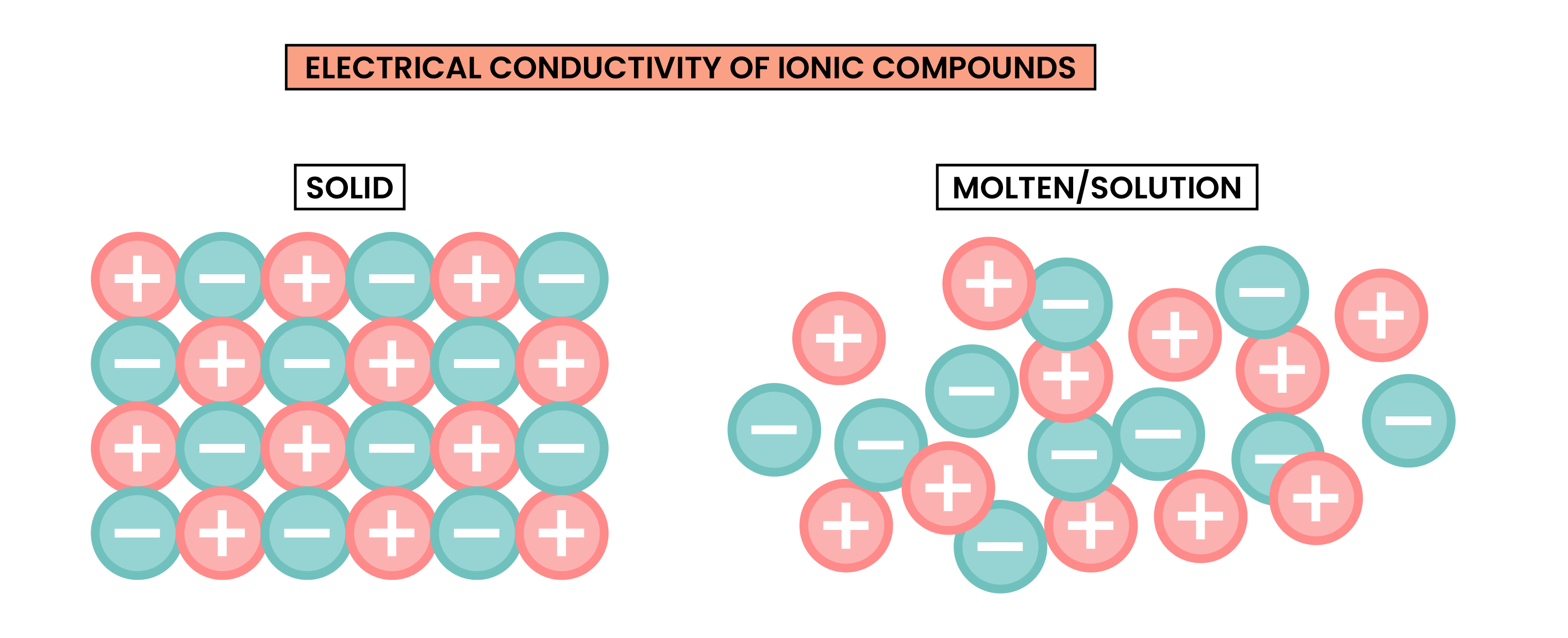
1.6.7 Know that ionic compounds do not conduct electricity when solid, but do conduct electricity when molten and in aqueous solution
ELECTRICAL CONDUCTIVITY OF IONIC COMPOUNDS
For a substance to conduct electricity, there should be free-moving, charged particles, for example:
- Ions in solution
- Free electrons in metal