REVISION NOTES
IGCSE Edexcel Chemistry
1.8 Metallic Bonding
1.8.1C Know how to represent a metallic lattice by a 2-D diagram
RECALL
- Metals tend to lose electrons to form full electron shells
- They form positive cations
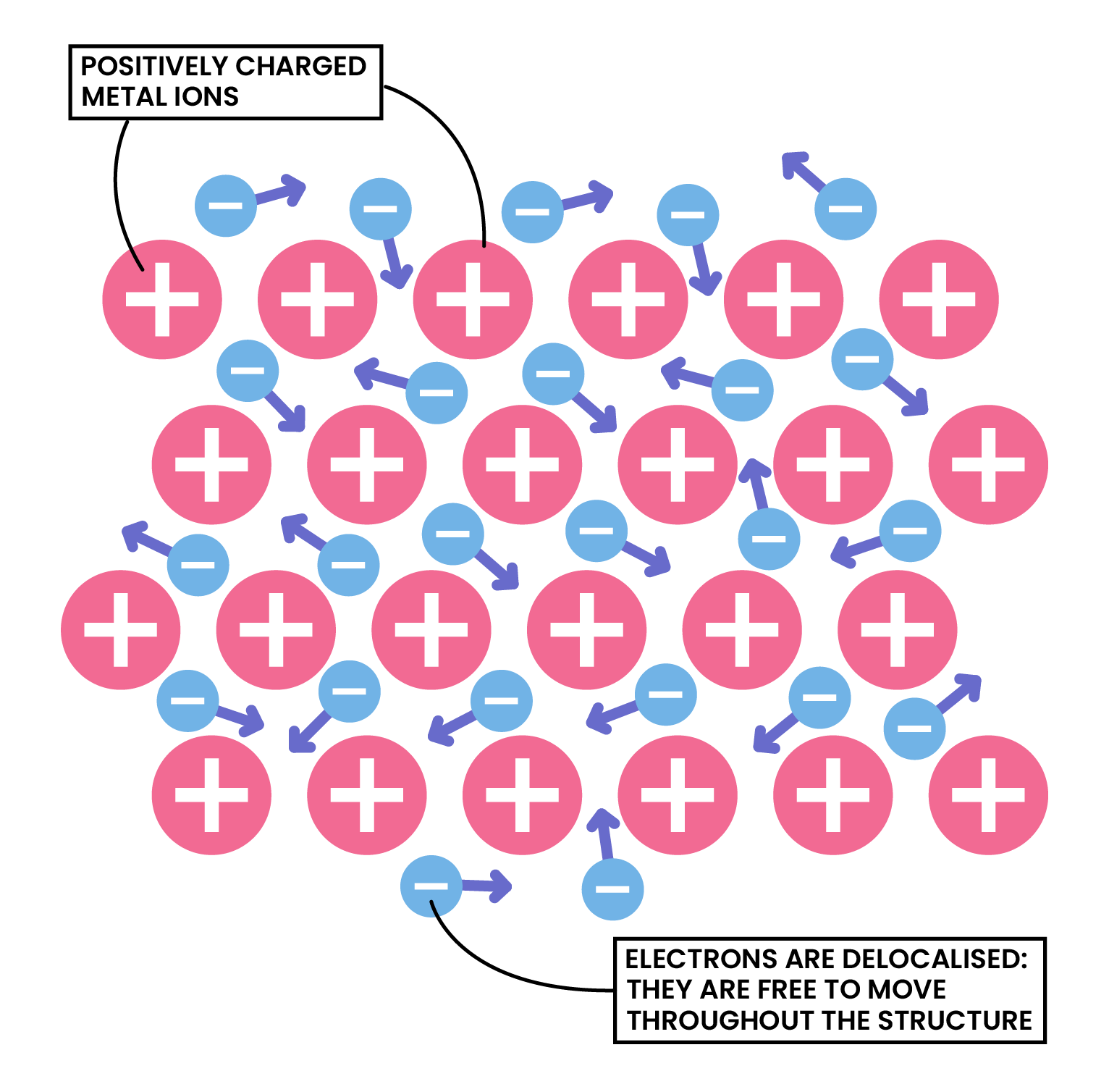
Metal ions are held together strongly by metallic bonding
- Atoms are arranged in a lattice
- Electrons lost by the metal atoms are delocalised
- The delocalised electrons move freely between the positive metal ions like a sea of electrons
1.8.2C Understand metallic bonding in terms of electrostatic attractions
Metallic Bonding
Electrostatic attraction forces between positive metal ions lattice and negative delocalised electrons
1.8.3C Explain typical physical properties of metals, including electrical conductivity and malleability
- Metals have high melting and boiling points due to strong metallic bonds that require high levels of energy to break.
- Metals are malleable (can be hammered into thin sheets) as layers of positive ions can slide over each other without breaking the metallic bonds.

