REVISION NOTES
IGCSE Edexcel Physics
5.2 Change of State
5.2.1P Explain why heating a system will change the energy stored within the system and raise its temperature or produce changes of state
- Heating a system will increase the energy in the system
- Due to heat being a form of energy
- Heating a system will raise the temperature of the system
- Once the temperature reaches a certain point the energy will be high enough so that the substance can change state
- In a solid, particles gain enough energy to overcome the forces of attraction and move over one another, becoming a liquid
- In a liquid, particles gain enough energy to move away from each other, becoming a gas
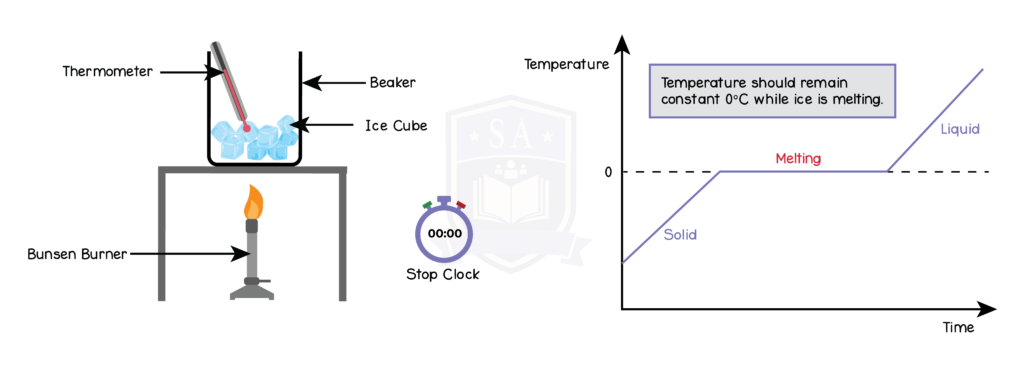
5.2.2P Describe the changes that occur when a solid melts to form a liquid, and when a liquid evaporates or boils to form a gas

5.2.3P Describe the arrangement and motion of particles in solids, liquids and gases

5.2.4P Practical: obtain a temperature–time graph to show the constant temperature during a change of state
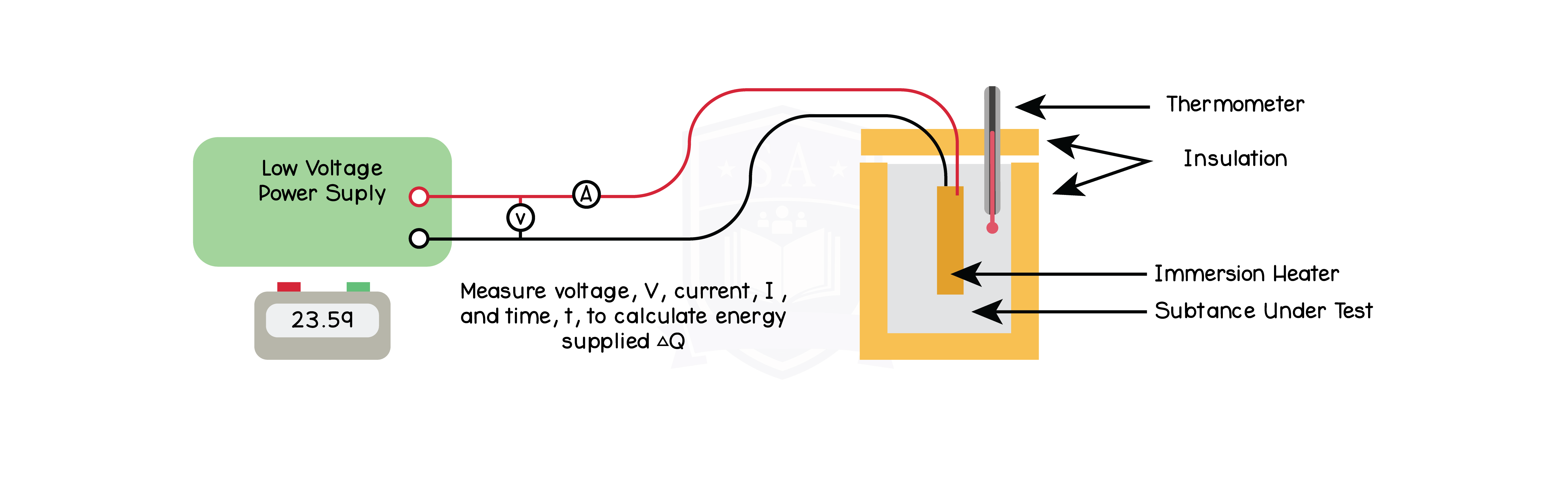
5.2.5P Know that specific heat capacity is the energy required to change the temperature of an object by one degree Celsius per kilogram of mass (J/kg °C)
5.2.6P Use the equation:
change in thermal energy = mass × specific heat capacity × change in temperature

5.2.7P Practical: investigate the specific heat capacity of materials including water and some solids